"VSports注册入口" High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases
- PMID: 22234969
- PMCID: PMC4610360
- DOI: 10.1002/hep.25572
High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases (V体育官网入口)
V体育官网 - Abstract
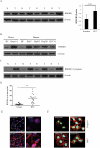
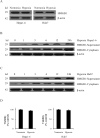
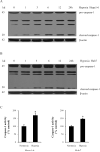
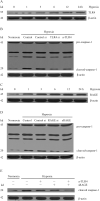
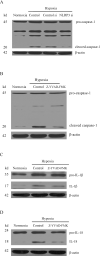
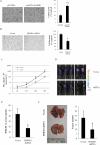
Hypoxia is often found in solid tumors and is associated with tumor progression and poor clinical outcomes. The exact mechanisms related to hypoxia-induced invasion and metastasis remain unclear. We elucidated the mechanism by which the nuclear-damage-associated molecular pattern molecule, high-mobility group box 1 (HMGB1), released under hypoxic stress, can induce an inflammatory response to promote invasion and metastasis in hepatocellular carcinoma (HCC) cells. Caspase-1 activation was found to occur in hypoxic HCC cells in a process that was dependent on the extracellular release of HMGB1 and subsequent activation of both Toll-like receptor 4 (TLR4)- and receptor for advanced glycation endproducts (RAGE)-signaling pathways. Downstream from hypoxia-induced caspase-1 activation, cleavage and release of proinflammatory cytokines interleukin (IL)-1β and -18 occurred. We further demonstrate that overexpression of HMGB1 or treatment with recombinant HMGB1 enhanced the invasiveness of HCC cells, whereas stable knockdown of HMGB1 remarkably reduced HCC invasion. Moreover, in a murine model of HCC pulmonary metastasis, stable knockdown of HMGB1 suppressed HCC invasion and metastasis. VSports手机版.
Conclusion: These results suggest that in hypoxic HCC cells, HMGB1 activates TLR4- and RAGE-signaling pathways to induce caspase-1 activation with the subsequent production of multiple inflammatory mediators, which, in turn, promote cancer invasion and metastasis. V体育安卓版.
Copyright © 2012 American Association for the Study of Liver Diseases V体育ios版. .
"VSports app下载" Figures


VSports app下载 - References
-
- Rampone B, Schiavone B, Confuorto G. Current management of hepatocellular cancer. Curr Oncol Rep. 2010;12:186–192. - PubMed
-
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. - PubMed
-
- Talwalkar JA, Gores GJ. Diagnosis and staging of hepatocellular carcinoma. Gastroenterology. 2004;127:S126–132. - PubMed
-
- Lu SC, Mato JM. Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol. 2005;35:227–234. - PubMed
-
- Di Bisceglie AM, Simpson LH, Lotze MT, Hoofnagle JH. Development of hepatocellular carcinoma among patients with chronic liver disease due to hepatitis C viral infection. J Clin Gastroenterol. 1994;19:222–226. - PubMed
Publication types
MeSH terms
- "V体育官网" Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
- V体育官网 - Actions
"V体育官网入口" Substances
- Actions (VSports注册入口)
- Actions (VSports)
Grants and funding
LinkOut - more resources (V体育官网入口)
Full Text Sources
Other Literature Sources
"V体育官网" Medical
