"VSports手机版" Balancing neural crest cell intrinsic processes with those of the microenvironment in Tcof1 haploinsufficient mice enables complete enteric nervous system formation
- PMID: 22228097
- PMCID: PMC3313795
- DOI: "V体育安卓版" 10.1093/hmg/ddr611
Balancing neural crest cell intrinsic processes with those of the microenvironment in Tcof1 haploinsufficient mice enables complete enteric nervous system formation
Abstract
The enteric nervous system (ENS) comprises a complex neuronal network that regulates peristalsis of the gut wall and secretions into the lumen. The ENS is formed from a multipotent progenitor cell population called the neural crest, which is derived from the neuroepithelium. Neural crest cells (NCCs) migrate over incredible distances to colonize the entire length of the gut and during their migration they must survive, proliferate and ultimately differentiate VSports手机版. The absence of an ENS from variable lengths of the colon results in Hirschsprung's disease (HSCR) or colonic aganglionosis. Mutations in about 12 different genes have been identified in HSCR patients but the complex pattern of inheritance and variable penetrance suggests that additional genes or modifiers must be involved in the etiology and pathogenesis of this disease. We discovered that Tcof1 haploinsufficiency in mice models many of the early features of HSCR. Neuroepithelial apoptosis diminished the size of the neural stem cell pool resulting in reduced NCC numbers and their delayed migration along the gut from E10. 5 to E14. 5. Surprisingly however, we observe continued and complete colonization of the entire colon throughout E14. 5-E18. 5, a period in which the gut is considered to be non- or less-permissive to NCC. Thus, we reveal for the first time that reduced NCC progenitor numbers and delayed migration do not unequivocally equate with a predisposition for the pathogenesis of HSCR. In fact, these deficiencies can be overcome by balancing NCC intrinsic processes of proliferation and differentiation with extrinsic influences of the gut microenvironment. .
Figures
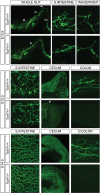
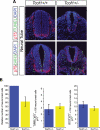

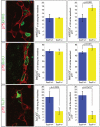
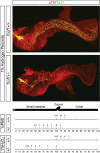

References
-
- Le Douarin N.M., Teillet M.A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 1973;30:31–48. - PubMed
-
- Yntema C.L., Hammond W.S. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J. Comp. Neurol. 1954;101:515–541. doi:10.1002/cne.901010212. - DOI - PubMed
-
- Durbec P.L., Larsson-Blomberg L.B., Schuchardt A., Costantini F., Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. - PubMed
-
- Kapur R.P., Yost C., Palmiter R.D. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992;116:167–175. - PubMed
-
- Natarajan D., Marcos-Gutierrez C., Pachnis V., de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. - PubMed
"VSports" Publication types
- Actions (VSports)
- "VSports在线直播" Actions
MeSH terms
- Actions (V体育2025版)
- Actions (V体育ios版)
- "V体育ios版" Actions
- "V体育官网入口" Actions
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
- Actions (V体育ios版)
- VSports app下载 - Actions
- "V体育2025版" Actions
"VSports注册入口" Substances
- Actions (VSports手机版)
- "V体育2025版" Actions
- Actions (VSports在线直播)
Grants and funding
LinkOut - more resources
Full Text Sources
V体育2025版 - Molecular Biology Databases

