Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6
- PMID: 22209248
- PMCID: PMC3257960
- DOI: "V体育2025版" 10.1016/j.ajhg.2011.11.020
Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6
Abstract
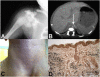
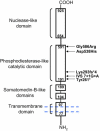
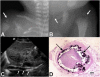
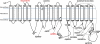
Spontaneous pathologic arterial calcifications in childhood can occur in generalized arterial calcification of infancy (GACI) or in pseudoxanthoma elasticum (PXE). GACI is associated with biallelic mutations in ENPP1 in the majority of cases, whereas mutations in ABCC6 are known to cause PXE VSports手机版. However, the genetic basis in subsets of both disease phenotypes remains elusive. We hypothesized that GACI and PXE are in a closely related spectrum of disease. We used a standardized questionnaire to retrospectively evaluate the phenotype of 92 probands with a clinical history of GACI. We obtained the ENPP1 genotype by conventional sequencing. In those patients with less than two disease-causing ENPP1 mutations, we sequenced ABCC6. We observed that three GACI patients who carried biallelic ENPP1 mutations developed typical signs of PXE between 5 and 8 years of age; these signs included angioid streaks and pseudoxanthomatous skin lesions. In 28 patients, no disease-causing ENPP1 mutation was found. In 14 of these patients, we detected pathogenic ABCC6 mutations (biallelic mutations in eight patients, monoallelic mutations in six patients). Thus, ABCC6 mutations account for a significant subset of GACI patients, and ENPP1 mutations can also be associated with PXE lesions in school-aged children. Based on the considerable overlap of genotype and phenotype of GACI and PXE, both entities appear to reflect two ends of a clinical spectrum of ectopic calcification and other organ pathologies, rather than two distinct disorders. ABCC6 and ENPP1 mutations might lead to alterations of the same physiological pathways in tissues beyond the artery. .
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved. V体育安卓版.
VSports - Figures


References
-
- Rutsch F., Vaingankar S., Johnson K., Goldfine I., Maddux B., Schauerte P., Kalhoff H., Sano K., Boisvert W.A., Superti-Furga A., Terkeltaub R. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am. J. Pathol. 2001;158:543–554. - PMC - PubMed
-
- Moran J.J. Idiopathic arterial calcification of infancy: A clinicopathologic study. Pathol. Annu. 1975;10:393–417. - PubMed
-
- Morton R. Idiopathic arterial calcification in infancy. Histopathology. 1978;2:423–432. - PubMed
-
- Ramjan K.A., Roscioli T., Rutsch F., Sillence D., Munns C.F. Generalized arterial calcification of infancy: Treatment with bisphosphonates. Nat. Clin. Pract. Endocrinol. Metab. 2009;5:167–172. - PubMed
Publication types
- Actions (VSports最新版本)
MeSH terms
- Actions (V体育官网入口)
- "VSports app下载" Actions
- Actions (V体育ios版)
- "VSports最新版本" Actions
- Actions (V体育ios版)
- "VSports在线直播" Actions
- "V体育ios版" Actions
Substances
- "VSports app下载" Actions
Supplementary concepts
V体育平台登录 - Grants and funding
"V体育安卓版" LinkOut - more resources
VSports app下载 - Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous (V体育安卓版)

