Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis
- PMID: 22207581
- PMCID: PMC3330778
- DOI: 10.1152/ajpgi.00223.2011 (V体育安卓版)
V体育平台登录 - Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis
VSports - Abstract
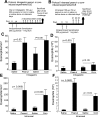
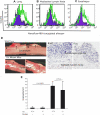
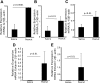
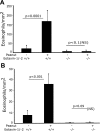
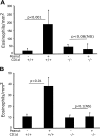
Eosinophilic esophagitis (EoE) is a recently recognized inflammatory disorder driven by food hypersensitivity; however, the specific foods and mechanisms involved are unclear. In patients with EoE, we have found that hypersensitivities to corn and peanuts are the most common. Accordingly, we sensitized and exposed mice either intranasally or intragastrically with corn or peanut extract or saline. Esophageal eosinophilia, the genes of eosinophil-directed cytokines, and allergen-induced antibodies were examined in mice challenged with corn or peanut extract or saline. A high number of esophageal lamina propria eosinophils as well as eosinophilic microabscesses, intraepithelial eosinophils, extracellular eosinophilic granules, thickened and disrupted epithelial mucosa, and mast cell hyperplasia were observed in the esophagus of peanut or corn allergen-challenged mice. Mechanistic analysis indicated that para-esophageal lymph nodes might be critical in the trafficking of eosinophils to the esophagus and in EoE association to airway eosinophilia VSports手机版. Furthermore, experimentation with gene-targeted mice revealed that peanut allergen-induced EoE was dependent on eotaxin and invariant natural killer T (iNKT) cells, as CD1d and eotaxin-1/2 gene-deficient mice were protected from disease induction. Thus we provide evidence that para-esophageal lymph nodes are involved in food- or aeroallergen-induced eosinophilia and patchy EoE pathogenesis, likely a mechanism dependent on eotaxins and iNKT cells. .
"VSports app下载" Figures


References
-
- Arora AS, Yamazaki K. Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol 2: 523–530, 2004. - PubMed (V体育安卓版)
-
- Bachurski CJ, Pryhuber GS, Glasser SW, Kelly SE, Whitsett JA. Tumor necrosis factor-alpha inhibits surfactant protein C gene transcription. J Biol Chem 270: 19402–19407, 1995. - PubMed
-
- Becky Kelly EA, Busse WW, Jarjour NN. Inhaled budesonide decreases airway inflammatory response to allergen. Am J Respir Crit Care Med 162: 883–890, 2000. - PubMed
-
- Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 116: 536–547, 2006. - PMC - PubMed
-
- Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics 79: 683–688, 1987. - PubMed
Publication types
"V体育ios版" MeSH terms
- V体育官网入口 - Actions
- Actions (V体育2025版)
- "V体育ios版" Actions
- V体育安卓版 - Actions
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- Actions (VSports手机版)
- VSports在线直播 - Actions
- VSports - Actions
- "VSports app下载" Actions
- Actions (VSports注册入口)
Substances
- "VSports app下载" Actions
Grants and funding
LinkOut - more resources
"VSports" Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

