"V体育ios版" Hydrodynamic cellular volume changes enable glioma cell invasion
- PMID: 22114291
- PMCID: PMC3253353
- DOI: V体育ios版 - 10.1523/JNEUROSCI.3938-11.2011
Hydrodynamic cellular volume changes enable glioma cell invasion
Abstract
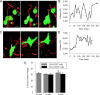
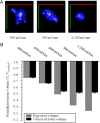
Malignant gliomas are highly invasive brain tumors that currently lack effective treatment. Unlike other cancers, gliomas do not metastasize via the vasculature but invade surrounding brain solely along extracellular routes, primarily moving along the vasculature and nerve tracts. This study uses several model systems to visualize and quantitatively assess cell volume changes of human glioma cells invading within the brain's extracellular space of C. B. 17 severe combined immunodeficient (scid) mice and tumor cells invading in a modified Boyden chamber using three-dimensional multiphoton and confocal time-lapse microscopy. Regardless of model system used to quantitatively assess volume changes, invading glioma cells maximally decreased their volume by 30-35%, a value that was independent of barrier and cell size VSports手机版. Through osmotic challenges, we demonstrate that the observed cellular volume changes during invasion represent the smallest achievable cell volume and require glioma cells to release all free unbound cytoplasmic water. Water osmotically follows the release of Cl(-) through ion channels and cotransporters and blockade of Cl(-) flux inhibits both volume changes and cell invasion. Hence, invading glioma cells use hydrodynamic volume changes to meet the spatial constraints imposed within the brain, using essentially all free, unbound cytoplasmic water to maximally alter their volume as they invade. .
Figures




V体育2025版 - References
-
- Abbas L, Whitfield TT. Nkcc1 (Slc12a2) is required for the regulation of endolymph volume in the otic vesicle and swim bladder volume in the zebrafish larva. Development. 2009;136:2837–2848. - "V体育ios版" PMC - PubMed
-
- Berens ME, Rief MD, Loo MA, Giese A. The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Metastasis. 1994;12:405–415. - PubMed (V体育官网)
-
- Bigner DD, Bigner SH, Pontén J, Westermark B, Mahaley MS, Ruoslahti E, Herschman H, Eng LF, Wikstrand CJ. Heterogeneity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981;40:201–229. - V体育官网 - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms (V体育2025版)
- "V体育ios版" Actions
- "V体育官网入口" Actions
- Actions (V体育官网)
- VSports - Actions
- Actions (V体育ios版)
- VSports最新版本 - Actions
- Actions (VSports注册入口)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
