Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection (V体育官网入口)
- PMID: 22034068
- PMCID: VSports在线直播 - PMC3288456
- DOI: VSports - 10.1002/art.33444
Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection
Abstract
Objective: Mechanical injury induces cell death in cartilage and triggers a remodeling process that ultimately can manifest as osteoarthritis VSports手机版. Autophagy is a process for turnover of intracellular organelles and macromolecules that protects cells during stress responses. This study was undertaken to determine changes in and functions of autophagy following mechanical injury to cartilage. .
Methods: Bovine and human cartilage explants were subjected to mechanical impact (40% strain for 500 msec). Cell viability, sulfated glycosaminoglycan (sGAG) release, and changes in the levels of the autophagy markers ULK1, beclin 1, and microtubule-associated protein 1 light chain 3 (LC3) were evaluated. Cartilage explants were treated with the mammalian target of rapamycin complex 1 (mTORC-1) inhibitor and the autophagy inducer rapamycin and tested for protective effects against mechanical injury. Explants were also treated with the cell death inducers nitric oxide and tumor necrosis factor α (TNFα) plus actinomycin D, and the proinflammatory cytokine interleukin-1α (IL-1α) V体育安卓版. .
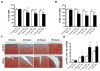
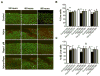
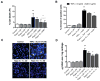
Results: Mechanical injury induced cell death and loss of sGAG in a time-dependent manner. This was associated with significantly decreased ULK1, beclin 1, and LC3 expression in the cartilage superficial zone (P < 0. 05) 48 hours after injury. The levels of LC3-II were increased 24 hours after injury but decreased at 48 and 96 hours. Rapamycin enhanced expression of autophagy regulators and prevented cell death and sGAG loss in mechanically injured explants. Rapamycin also protected against cell death induced by sodium nitroprusside and TNFα plus actinomycin D and prevented sGAG loss induced by IL-1α V体育ios版. .
Conclusion: Our findings indicate that mechanical injury leads to suppression of autophagy, predominantly in the superficial zone where most of the cell death occurs VSports最新版本. Pharmacologic inhibition of mTORC-1, at least in part by enhancement of autophagy, prevents cell and matrix damage, suggesting a novel approach for chondroprotection. .
Copyright © 2012 by the American College of Rheumatology. V体育平台登录.
Figures


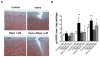
References
-
- Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–93. - PubMed
-
- Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–301. - PubMed
-
- Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60:2985–96. - "V体育官网入口" PubMed
Publication types
MeSH terms
- "VSports注册入口" Actions
- V体育官网 - Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- "V体育ios版" Actions
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- "V体育官网入口" Actions
Substances
- VSports - Actions
- Actions (V体育安卓版)
- "VSports最新版本" Actions
- V体育ios版 - Actions
Grants and funding
"VSports app下载" LinkOut - more resources
"VSports最新版本" Full Text Sources
Other Literature Sources
Research Materials

