mTOR drives its own activation via SCF(βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR
- PMID: 22017875
- PMCID: PMC3229299 (VSports app下载)
- DOI: 10.1016/j.molcel.2011.08.030
mTOR drives its own activation via SCF(βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR
"VSports在线直播" Abstract
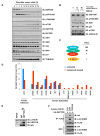
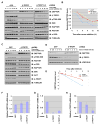
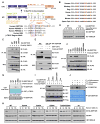
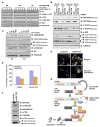
The activities of both mTORC1 and mTORC2 are negatively regulated by their endogenous inhibitor, DEPTOR VSports手机版. As such, the abundance of DEPTOR is a critical determinant in the activity status of the mTOR network. DEPTOR stability is governed by the 26S-proteasome through a largely unknown mechanism. Here we describe an mTOR-dependent phosphorylation-driven pathway for DEPTOR destruction via SCF(βTrCP). DEPTOR phosphorylation by mTOR in response to growth signals, and in collaboration with casein kinase I (CKI), generates a phosphodegron that binds βTrCP. Failure to degrade DEPTOR through either degron mutation or βTrCP depletion leads to reduced mTOR activity, reduced S6 kinase activity, and activation of autophagy to reduce cell growth. This work expands the current understanding of mTOR regulation by revealing a positive feedback loop involving mTOR and CKI-dependent turnover of its inhibitor, DEPTOR, suggesting that misregulation of the DEPTOR destruction pathway might contribute to aberrant activation of mTOR in disease. .
Copyright © 2011 Elsevier Inc. All rights reserved. V体育安卓版.
VSports在线直播 - Conflict of interest statement
Conflict of Interest Statement: J. W. H V体育ios版. is a consultant for Millennium Pharmaceuticals.
Figures


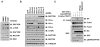
Comment in
-
Cell signalling: mTOR targets its own inhibitor.Nat Rev Mol Cell Biol. 2011 Nov 9;12(12):769. doi: 10.1038/nrm3229. Nat Rev Mol Cell Biol. 2011. PMID: 22068633 No abstract available.
References
-
- Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:pe27. - V体育官网入口 - PubMed
-
- Boehm JS, Hession MT, Bulmer SE, Hahn WC. Transformation of human and murine fibroblasts without viral oncoproteins. Mol Cell Biol. 2005;25:6464–6474. - "VSports在线直播" PMC - PubMed
-
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. - PubMed
-
- Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. - "V体育平台登录" PMC - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- Actions (VSports)
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "VSports手机版" Actions
- VSports app下载 - Actions
- "VSports在线直播" Actions
Substances
- "V体育2025版" Actions
- "V体育官网入口" Actions
- V体育官网入口 - Actions
- "V体育ios版" Actions
"VSports最新版本" Grants and funding
- GM0089877/GM/NIGMS NIH HHS/United States
- R01 CA122099/CA/NCI NIH HHS/United States
- V体育2025版 - AG011085/AG/NIA NIH HHS/United States
- K01 AG041218/AG/NIA NIH HHS/United States
- R01 GM095567/GM/NIGMS NIH HHS/United States
- GM054137/GM/NIGMS NIH HHS/United States
- R01 GM089763/GM/NIGMS NIH HHS/United States (VSports最新版本)
- VSports最新版本 - CA122099/CA/NCI NIH HHS/United States
- R01 GM054137/GM/NIGMS NIH HHS/United States
- R01 GM056203/GM/NIGMS NIH HHS/United States
- R01 AG011085/AG/NIA NIH HHS/United States
- GM089763/GM/NIGMS NIH HHS/United States
V体育2025版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育安卓版)
Research Materials
Miscellaneous

