"V体育官网" Estimating treatment effects for individual patients based on the results of randomised clinical trials
- PMID: 21968126
- PMCID: PMC3184644
- DOI: 10.1136/bmj.d5888
Estimating treatment effects for individual patients based on the results of randomised clinical trials
Abstract
Objectives: To predict treatment effects for individual patients based on data from randomised trials, taking rosuvastatin treatment in the primary prevention of cardiovascular disease as an example, and to evaluate the net benefit of making treatment decisions for individual patients based on a predicted absolute treatment effect VSports手机版. .
Setting: As an example, data were used from the Justification for the Use of Statins in Prevention (JUPITER) trial, a randomised controlled trial evaluating the effect of rosuvastatin 20 mg daily versus placebo on the occurrence of cardiovascular events (myocardial infarction, stroke, arterial revascularisation, admission to hospital for unstable angina, or death from cardiovascular causes). Population 17,802 healthy men and women who had low density lipoprotein cholesterol levels of less than 3. 4 mmol/L and high sensitivity C reactive protein levels of 2. 0 mg/L or more V体育安卓版. .
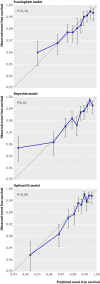
Methods: Data from the Justification for the Use of Statins in Prevention trial were used to predict rosuvastatin treatment effect for individual patients based on existing risk scores (Framingham and Reynolds) and on a newly developed prediction model V体育ios版. We compared the net benefit of prediction based rosuvastatin treatment (selective treatment of patients whose predicted treatment effect exceeds a decision threshold) with the net benefit of treating either everyone or no one. .
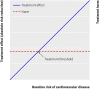
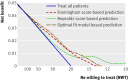
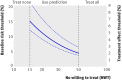
Results: The median predicted 10 year absolute risk reduction for cardiovascular events was 4. 4% (interquartile range 2. 6-7. 0%) based on the Framingham risk score, 4. 2% (2. 5-7. 1%) based on the Reynolds score, and 3. 9% (2. 5-6. 1%) based on the newly developed model (optimal fit model). Prediction based treatment was associated with more net benefit than treating everyone or no one, provided that the decision threshold was between 2% and 7%, and thus that the number willing to treat (NWT) to prevent one cardiovascular event over 10 years was between 15 and 50. VSports最新版本.
Conclusions: Data from randomised trials can be used to predict treatment effect in terms of absolute risk reduction for individual patients, based on a newly developed model or, if available, existing risk scores. The value of such prediction of treatment effect for medical decision making is conditional on the NWT to prevent one outcome event V体育平台登录. Trial registration number Clinicaltrials. gov NCT00239681. .
V体育安卓版 - Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www. icmje. org/coi_disclosure. pdf (available on request from the corresponding author) and declare: PMR is the principal investigator of the investigator initiated Justification for the Use of Statins in Prevention trial, which was funded by AstraZeneca (Wilmington, Delaware). PMR received grant support from Novartis and Roche; consulting fees from Siemens Medical Systems, ISIS, and Vascular Biogenetics; and is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to Siemens Medical Systems (Erlangen, Germany) and AstraZeneca. FLJV’s department receives grant support from Merck, the Netherlands Organisation for Health Research and Development, and the Catharijne Foundation Utrecht; and speaker fees from Merck and AstraZeneca. JAND, AMJW, NPP, EWS, YvdG, and NRC have no relationships with industry that might have an interest in the submitted work in the previous three years. All authors have no non-financial interests that may be relevant to the submitted work VSports注册入口.
Figures

References
-
- Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol 2006;6:18. - V体育2025版 - PMC - PubMed
-
- Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA 2007;298:1209-12. - PubMed
-
- Rothwell PM. Can overall results of clinical trials be applied to all patients? Lancet 1995;345:1616-9. - PubMed
"V体育平台登录" Publication types
- Actions (V体育官网入口)
- "V体育2025版" Actions
MeSH terms
- VSports最新版本 - Actions
- VSports手机版 - Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
- "V体育安卓版" Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
- V体育平台登录 - Actions
- VSports app下载 - Actions
- "V体育官网入口" Actions
- VSports app下载 - Actions
- VSports手机版 - Actions
- "V体育2025版" Actions
- "V体育2025版" Actions
- VSports - Actions
"VSports在线直播" Substances
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- VSports最新版本 - Actions
Associated data
"V体育官网入口" LinkOut - more resources
Full Text Sources
"VSports最新版本" Medical
Research Materials
