Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish
- PMID: 21968100
- PMCID: PMC3303755
- DOI: 10.1016/j.ydbio.2011.09.010
VSports手机版 - Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish
Abstract
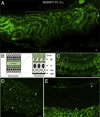
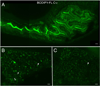
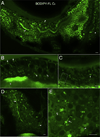
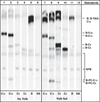
Lipids are essential for cellular function as sources of fuel, critical signaling molecules and membrane components. Deficiencies in lipid processing and transport underlie many metabolic diseases. To better understand metabolic function as it relates to disease etiology, a whole animal approach is advantageous, one in which multiple organs and cell types can be assessed simultaneously in vivo. Towards this end, we have developed an assay to visualize fatty acid (FA) metabolism in larval zebrafish (Danio rerio). The method utilizes egg yolk liposomes to deliver different chain length FA analogs (BODIPY-FL) to six day-old larvae. Following liposome incubation, larvae accumulate the analogs throughout their digestive organs, providing a comprehensive readout of organ structure and physiology VSports手机版. Using this assay we have observed that different chain length FAs are differentially transported and metabolized by the larval digestive system. We show that this assay can also reveal structural and metabolic defects in digestive mutants. Because this labeling technique can be used to investigate digestive organ morphology and function, we foresee its application in diverse studies of organ development and physiology. .
Copyright © 2011 Elsevier Inc. All rights reserved V体育安卓版. .
Figures



V体育2025版 - References
-
- Andre M, Ando S, Ballagny C, Durliat M, Poupard G, Briancon C, Babin PJ. Intestinal fatty acid binding protein gene expression reveals the cephalocaudal patterning during zebrafish gut morphogenesis. Int. J. Dev. Biol. 2000;44(2):249–252. - PubMed
-
- Atshaves BP, Storey SM, Huang H, Schroeder F. Liver fatty acid binding protein expression enhances branched-chain fatty acid metabolism. Mol. Cell. Biochem. 2004;259(1–2):115–129. - PubMed
-
- Bach AM, Hann LE, Brown KT, Getrajdman GI, Herman SK, Fong Y, Blumgart LH. Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology. 1996;201(1):149–154. - PubMed
-
- Benzonana G, Desnuelle P. Kinetic study of the action of pancreatic lipase on emulsified triglycerides. Enzymology assay in heterogeneous medium. Biochim. Biophys. Acta. 1965;105(1):121–136. - PubMed
MeSH terms
- Actions (V体育2025版)
- "VSports手机版" Actions
- "V体育官网" Actions
- "V体育官网" Actions
- "V体育2025版" Actions
- Actions (V体育ios版)
- "VSports在线直播" Actions
- VSports app下载 - Actions
- VSports手机版 - Actions
- V体育平台登录 - Actions
Substances
- Actions (V体育官网)
- VSports最新版本 - Actions
- "VSports最新版本" Actions
- Actions (VSports在线直播)
Grants and funding
LinkOut - more resources
VSports app下载 - Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

