Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity
- PMID: 21909099
- PMCID: PMC4208311
- DOI: 10.1038/ncb2332
Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity
"VSports" Erratum in
- Nat Cell Biol. 2011 Dec;13(12):1467
Abstract
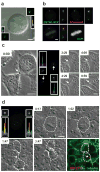
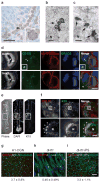
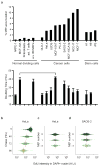
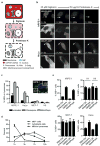
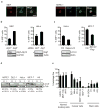
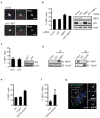
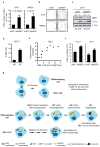
The midbody is a singular organelle formed between daughter cells during cytokinesis and required for their final separation. Midbodies persist in cells long after division as midbody derivatives (MB(d)s), but their fate is unclear. Here we show that MB(d)s are inherited asymmetrically by the daughter cell with the older centrosome. They selectively accumulate in stem cells, induced pluripotent stem cells and potential cancer 'stem cells' in vivo and in vitro. MB(d) loss accompanies stem-cell differentiation, and involves autophagic degradation mediated by binding of the autophagic receptor NBR1 to the midbody protein CEP55. Differentiating cells and normal dividing cells do not accumulate MB(d)s and possess high autophagic activity. Stem cells and cancer cells accumulate MB(d)s by evading autophagosome encapsulation and exhibit low autophagic activity. MB(d) enrichment enhances reprogramming to induced pluripotent stem cells and increases the in vitro tumorigenicity of cancer cells. These results indicate unexpected roles for MB(d)s in stem cells and cancer 'stem cells' VSports手机版. .
Figures

Comment in
-
Organelle dynamics: Inheritance for pluripotency.Nat Rev Mol Cell Biol. 2011 Oct 12;12(11):690-1. doi: 10.1038/nrm3216. Nat Rev Mol Cell Biol. 2011. PMID: 21993293 No abstract available.
References
-
- Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–66. - PubMed
-
- Neumüller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99. - "VSports在线直播" PMC - PubMed
-
- Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu Rev Cell Dev Biol. 2005;21:411–34. - VSports app下载 - PubMed
-
- Wang X, et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–55. - PMC (V体育官网) - PubMed
"V体育平台登录" Publication types
MeSH terms
- "VSports最新版本" Actions
- "V体育官网" Actions
- Actions (VSports)
- Actions (V体育安卓版)
- Actions (VSports)
- Actions (V体育官网入口)
- Actions (VSports)
- Actions (VSports手机版)
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- VSports在线直播 - Actions
- Actions (VSports手机版)
- V体育2025版 - Actions
- V体育2025版 - Actions
Substances
- "V体育2025版" Actions
- "V体育平台登录" Actions
- V体育安卓版 - Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育2025版" Molecular Biology Databases
Research Materials
Miscellaneous

