TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding
- PMID: 21829704
- PMCID: PMC3148248
- DOI: 10.1371/journal.pone.0023132
TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding
Abstract
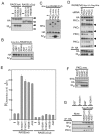
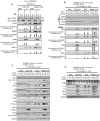
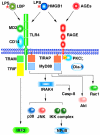
The receptor for advanced glycation end products (RAGE) is thought to be involved in the pathogenesis of a broad range of inflammatory, degenerative and hyperproliferative diseases. It binds to diverse ligands and activates multiple intracellular signaling pathways. Despite these pivotal functions, molecular events just downstream of ligand-activated RAGE have been surprisingly unknown. Here we show that the cytoplasmic domain of RAGE is phosphorylated at Ser391 by PKCζ upon binding of ligands VSports手机版. TIRAP and MyD88, which are known to be adaptor proteins for Toll-like receptor-2 and -4 (TLR2/4), bound to the phosphorylated RAGE and transduced a signal to downstream molecules. Blocking of the function of TIRAP and MyD88 largely abrogated intracellular signaling from ligand-activated RAGE. Our findings indicate that functional interaction between RAGE and TLRs coordinately regulates inflammation, immune response and other cellular functions. .
Conflict of interest statement
Competing Interests: Akira Motoyama and Toshihiko Hibino are employees of the Shiseido Research Center. There are no patents, products in development or marketed products to declare V体育安卓版. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
VSports注册入口 - Figures


VSports app下载 - References
-
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. - VSports在线直播 - PubMed
-
- Schmidt AM, Hori O, Cao R, Yan SD, Brett J, et al. RAGE: a novel cellular receptor for advanced glycation end products. Diabetes. 1996;45(Suppl 3):S77–80. - PubMed
-
- Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. - PubMed
-
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. - PubMed (VSports)
Publication types
MeSH terms
- Actions (VSports最新版本)
- V体育官网入口 - Actions
- VSports最新版本 - Actions
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- VSports在线直播 - Actions
- V体育ios版 - Actions
- Actions (VSports手机版)
- Actions (VSports注册入口)
- "VSports app下载" Actions
- "VSports手机版" Actions
- V体育平台登录 - Actions
- Actions (V体育官网入口)
- "V体育官网" Actions
- V体育官网 - Actions
Substances
- "VSports" Actions
- VSports - Actions
- VSports在线直播 - Actions
- VSports最新版本 - Actions
- "V体育官网" Actions
- "VSports手机版" Actions
- Actions (VSports注册入口)
"VSports app下载" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

