Exploring the biology of lipid peroxidation-derived protein carbonylation
- PMID: 21812433
- PMCID: PMC3178011
- DOI: 10.1021/tx200169n
Exploring the biology of lipid peroxidation-derived protein carbonylation
Abstract
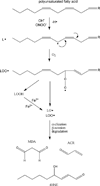
The sustained overproduction of reactive oxygen and nitrogen species results in an imbalance of cellular prooxidant-antioxidant systems and is implicated in numerous disease states, including alcoholic liver disease, cancer, neurological disorders, inflammation, and cardiovascular disease. The accumulation of reactive aldehydes resulting from sustained oxidative stress and lipid peroxidation is an underlying factor in the development of these pathologies. Determining the biochemical factors that elicit cellular responses resulting from protein carbonylation remains a key element to developing therapeutic approaches and ameliorating disease pathologies. This review details our current understanding of the generation of reactive aldehydes via lipid peroxidation resulting in protein carbonylation, focusing on pathophysiologic factors associated with 4-hydroxynonenal-protein modification VSports手机版. Additionally, an overview of in vitro and in vivo model systems used to study the physiologic impact of protein carbonylation is presented. Finally, an update of the methods commonly used in characterizing protein modification by reactive aldehydes provides an overview of isolation techniques, mass spectrometry, and computational biology. It is apparent that research in this area employing state-of-the-art proteomics, mass spectrometry, and computational biology is rapidly evolving, yielding foundational knowledge concerning the molecular mechanisms of protein carbonylation and its relation to a spectrum of diseases associated with oxidative stress. .
Figures
References
-
- Sies H. Oxidative Stress II. Oxidants and Antioxidants. London: Academic Press; 1991.
-
- Moller IM, Rogowska-Wrzesinska A, Rao RS. Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. J Proteomics. 2011 - PubMed
"VSports注册入口" Publication types
- "VSports最新版本" Actions
- V体育安卓版 - Actions
MeSH terms
- Actions (VSports app下载)
- Actions (V体育官网入口)
- "VSports手机版" Actions
"V体育ios版" Substances
- V体育平台登录 - Actions
- Actions (VSports)
"V体育官网入口" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

