VSports在线直播 - Taurine biosynthesis by neurons and astrocytes
- PMID: 21778230
- PMCID: V体育ios版 - PMC3173217
- DOI: 10.1074/jbc.M111.253344
Taurine biosynthesis by neurons and astrocytes
Abstract
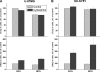
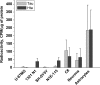
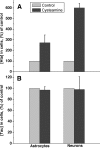
The physiological roles of taurine, a product of cysteine degradation and one of the most abundant amino acids in the body, remain elusive. Taurine deficiency leads to heart dysfunction, brain development abnormalities, retinal degradation, and other pathologies. The taurine synthetic pathway is proposed to be incomplete in astrocytes and neurons, and metabolic cooperation between these cell types is reportedly needed to complete the pathway. In this study, we analyzed taurine synthesis capability as reported by incorporation of radioactivity from [(35)S]cysteine into taurine, in primary murine astrocytes and neurons, and in several transformed cell lines (human (SH-SY5Y) and murine (N1E-115) neuroblastoma, human astrocytoma (U-87 MG and 1321 N1), and rat glioma (C6)). Extensive incorporation of radioactivity from [(35)S]cysteine into taurine was observed in rat glioma cells as well as in primary mouse astrocytes and neurons, establishing the presence of an intact taurine synthesis pathway in these cells VSports手机版. Interestingly, exposure of cells to cysteine or cysteamine resulted in elevated intracellular hypotaurine without a corresponding increase in taurine levels, suggesting that oxidation of hypotaurine limits taurine synthesis in cells. Consistent with its role as an organic osmolyte, taurine synthesis was stimulated under hypertonic conditions in neurons. .
"V体育ios版" Figures





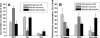

References
-
- Sumizu K. (1962) Biochim. Biophys. Acta 63, 210–212 - "V体育2025版" PubMed
-
- Oja S. S., Kontro P. (1981) Biochim. Biophys. Acta 677, 350–357 - PubMed
-
- Huxtable R. J. (1989) Prog. Neurobiol. 32, 471–533 - PubMed
-
- Fiori A., Costa M. (1969) Acta Vitaminol. Enzymol. 23, 204–207 - V体育2025版 - PubMed
-
- Ricci G., Dupré S., Federici G., Spoto G., Matarese R. M., Cavallini D. (1978) Physiol. Chem. Phys. 10, 435–441 - PubMed
"V体育官网" Publication types
MeSH terms
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- "V体育官网" Actions
- "V体育安卓版" Actions
- "VSports手机版" Actions
Substances
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources (V体育官网入口)
"V体育官网入口" Full Text Sources

