"V体育2025版" A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus
- PMID: 21708106
- PMCID: PMC3199295
- DOI: 10.1053/j.gastro.2011.06.051
A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus
"VSports" Abstract
Background & aims: T-helper (Th)17 cells that secrete interleukin (IL)-22 have immunomodulatory and protective properties in the liver and other tissues. IL-22 induces expression of proinflammatory genes but is also mitogenic and antiapoptotic in hepatocytes. Therefore, it could have multiple functions in the immune response to hepatitis B virus (HBV). VSports手机版.
Methods: We examined the role of IL-22 in regulating liver inflammation in HBV transgenic mice and measured levels of IL-22 in HBV-infected patients. V体育安卓版.
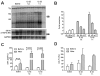
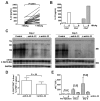
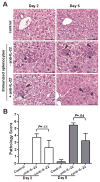
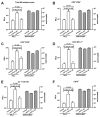
Results: In HBV transgenic mice, injection of a single dose of IL-22 increased hepatic expression of proinflammatory genes but did not directly inhibit virus replication. When splenocytes from HBV-immunized mice were transferred into HBV transgenic mice, the severity of the subsequent liver damage was ameliorated by neutralization of IL-22. In this model, IL-22 depletion did not affect interferon gamma-mediated noncytopathic inhibition of virus replication initiated by HBV-specific cytotoxic T cells, but it significantly inhibited recruitment of antigen-nonspecific inflammatory cells into the liver V体育ios版. In patients with acute HBV infections, the percentage of Th17 cells in peripheral blood and concentration of IL-22 in serum were significantly increased. .
Conclusions: IL-22 appears to be an important mediator of the inflammatory response following recognition of HBV by T cells in the liver. These findings might be relevant to the development of cytokine-based therapies for patients with HBV infection VSports最新版本. .
Copyright © 2011 AGA Institute. Published by Elsevier Inc V体育平台登录. All rights reserved. .
Conflict of interest statement
Figures
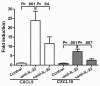
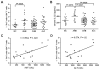
References
-
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. - PMC - PubMed
-
- Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin–22. Blood. 2009;113:4008–10. - PMC (VSports在线直播) - PubMed
-
- Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–9. - VSports最新版本 - PubMed
-
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. - PubMed
Publication types
- V体育ios版 - Actions
MeSH terms (VSports app下载)
- VSports app下载 - Actions
- V体育平台登录 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "V体育平台登录" Actions
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- "V体育官网入口" Actions
- Actions (VSports app下载)
Substances
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

