The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension
- PMID: 21697531
- PMCID: PMC3744110
- DOI: VSports最新版本 - 10.1126/scitranslmed.3002194
The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension
Abstract
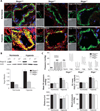
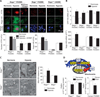
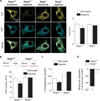
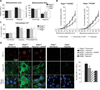
Pulmonary arterial hypertension (PAH) is caused by excessive proliferation of vascular cells, which occlude the lumen of pulmonary arteries (PAs) and lead to right ventricular failure. The cause of the vascular remodeling in PAH remains unknown, and the prognosis of PAH remains poor. Abnormal mitochondria in PAH PA smooth muscle cells (SMCs) suppress mitochondria-dependent apoptosis and contribute to the vascular remodeling. We hypothesized that early endoplasmic reticulum (ER) stress, which is associated with clinical triggers of PAH including hypoxia, bone morphogenetic protein receptor II mutations, and HIV/herpes simplex virus infections, explains the mitochondrial abnormalities and has a causal role in PAH VSports手机版. We showed in SMCs from mice that Nogo-B, a regulator of ER structure, was induced by hypoxia in SMCs of the PAs but not the systemic vasculature through activation of the ER stress-sensitive transcription factor ATF6. Nogo-B induction increased the distance between the ER and mitochondria and decreased ER-to-mitochondria phospholipid transfer and intramitochondrial calcium. In addition, we noted inhibition of calcium-sensitive mitochondrial enzymes, increased mitochondrial membrane potential, decreased mitochondrial reactive oxygen species, and decreased mitochondria-dependent apoptosis. Lack of Nogo-B in PASMCs from Nogo-A/B-/- mice prevented these hypoxia-induced changes in vitro and in vivo, resulting in complete resistance to PAH. Nogo-B in the serum and PAs of PAH patients was also increased. Therefore, triggers of PAH may induce Nogo-B, which disrupts the ER-mitochondria unit and suppresses apoptosis. This could rescue PASMCs from death during ER stress but enable the development of PAH through overproliferation. The disruption of the ER-mitochondria unit may be relevant to other diseases in which Nogo is implicated, such as cancer or neurodegeneration. .
VSports最新版本 - Figures


Comment in
-
Endoplasmic reticulum stress enters a Nogo zone.Sci Transl Med. 2011 Jun 22;3(88):88ps26. doi: 10.1126/scitranslmed.3002708. Sci Transl Med. 2011. PMID: 21697529
V体育安卓版 - References
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA, ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. - PubMed
-
- Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, Tognoni G. A meta-analysis of trials of pulmonary hypertension: A clinical condition looking for drugs and research methodology. Am. Heart J. 2007;153:1037–1047. - PubMed (V体育平台登录)
-
- Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S. Systematic review of trials using vasodilators in pulmonary arterial hypertension: Why a new approach is needed. Am. Heart J. 2010;159:245–257. - VSports手机版 - PubMed
-
- Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation. 2008;118:1486–1495. - PubMed
-
- Dromparis P, Sutendra G, Michelakis ED. The role of mitochondria in pulmonary vascular remodeling. J. Mol. Med. 2010;88:1003–1010. - PubMed
Publication types
- V体育ios版 - Actions
MeSH terms
- Actions (V体育2025版)
- "V体育官网" Actions
- "V体育ios版" Actions
- Actions (VSports手机版)
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- Actions (VSports注册入口)
- "V体育官网入口" Actions
- Actions (VSports app下载)
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- Actions (V体育官网)
Substances
- "VSports在线直播" Actions
- Actions (V体育2025版)
- Actions (VSports最新版本)
- Actions (V体育2025版)
Grants and funding
LinkOut - more resources
Full Text Sources (VSports手机版)
Other Literature Sources
"VSports最新版本" Medical

