Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey
- PMID: 21697530
- PMCID: PMC5027886
- DOI: 10.1126/scitranslmed.3002103 (VSports手机版)
V体育ios版 - Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey
Erratum in
- Sci Transl Med. 2011 Dec 7;3(112):112er9
"VSports" Abstract
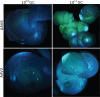
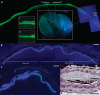
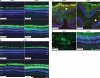
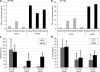
Gene therapy is emerging as a therapeutic modality for treating disorders of the retina. Photoreceptor cells are the primary cell type affected in many inherited diseases of retinal degeneration VSports手机版. Successfully treating these diseases with gene therapy requires the identification of efficient and safe targeting vectors that can transduce photoreceptor cells. One serotype of adeno-associated virus, AAV2, has been used successfully in clinical trials to treat a form of congenital blindness that requires transduction of the supporting cells of the retina in the retinal pigment epithelium (RPE). Here, we determined the dose required to achieve targeting of AAV2 and AAV8 vectors to photoreceptors in nonhuman primates. Transgene expression in animals injected subretinally with various doses of AAV2 or AAV8 vectors carrying a green fluorescent protein transgene was correlated with surgical, clinical, and immunological observations. Both AAV2 and AAV8 demonstrated efficient transduction of RPE, but AAV8 was markedly better at targeting photoreceptor cells. These preclinical results provide guidance for optimal vector and dose selection in future human gene therapy trials to treat retinal diseases caused by loss of photoreceptors. .
V体育官网 - Figures
References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
-
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. - PMC - PubMed
-
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. - PMC - PubMed
-
- Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 2010;29:335–375. - PubMed
-
- Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–359. - PubMed
V体育官网入口 - Publication types
- "VSports在线直播" Actions
- V体育ios版 - Actions
MeSH terms
- "VSports手机版" Actions
- VSports在线直播 - Actions
- V体育官网 - Actions
- "VSports app下载" Actions
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- Actions (VSports最新版本)
- VSports - Actions
- Actions (VSports在线直播)
- "VSports app下载" Actions
- Actions (V体育官网)
Substances
Grants and funding (VSports注册入口)
LinkOut - more resources
Full Text Sources
"V体育官网入口" Other Literature Sources
VSports在线直播 - Medical

