High-mobility group box 1 is essential for mitochondrial quality control
- PMID: 21641551
- PMCID: PMC3293110
- DOI: 10.1016/j.cmet.2011.04.008
High-mobility group box 1 is essential for mitochondrial quality control (V体育ios版)
Abstract
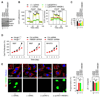
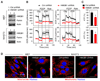
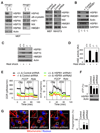
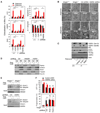
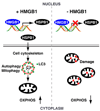
Mitochondria are organelles centrally important for bioenergetics as well as regulation of apoptotic death in eukaryotic cells. High-mobility group box 1 (HMGB1), an evolutionarily conserved chromatin-associated protein which maintains nuclear homeostasis, is also a critical regulator of mitochondrial function and morphology VSports手机版. We show that heat shock protein beta-1 (HSPB1 or HSP27) is the downstream mediator of this effect. Disruption of the HSPB1 gene in embryonic fibroblasts with wild-type HMGB1 recapitulates the mitochondrial fragmentation, deficits in mitochondrial respiration, and adenosine triphosphate (ATP) synthesis observed with targeted deletion of HMGB1. Forced expression of HSPB1 reverses this phenotype in HMGB1 knockout cells. Mitochondrial effects mediated by HMGB1 regulation of HSPB1 expression serve as a defense against mitochondrial abnormality, enabling clearance and autophagy in the setting of cellular stress. Our findings reveal an essential role for HMGB1 in autophagic surveillance with important effects on mitochondrial quality control. .
Copyright © 2011 Elsevier Inc. All rights reserved V体育安卓版. .
VSports注册入口 - Figures


References (VSports app下载)
-
- Arrigo AP. The cellular "networking" of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. - PubMed
-
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. - PubMed
-
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. - "VSports最新版本" PMC - PubMed
-
- Boldogh IR, Pon LA. Interactions of mitochondria with the actin cytoskeleton. Biochim Biophys Acta. 2006;1763:450–462. - V体育平台登录 - PubMed
VSports手机版 - Publication types
MeSH terms
- "V体育官网" Actions
- "VSports app下载" Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- VSports最新版本 - Actions
- Actions (V体育2025版)
- "VSports手机版" Actions
- "VSports手机版" Actions
- VSports - Actions
- "V体育官网" Actions
- V体育平台登录 - Actions
- V体育平台登录 - Actions
Substances
- VSports手机版 - Actions
- Actions (VSports app下载)
- VSports注册入口 - Actions
- Actions (VSports)
- "V体育官网入口" Actions
- Actions (VSports手机版)
"VSports最新版本" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
"V体育官网入口" Research Materials
Miscellaneous

