Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma
- PMID: 21636707
- PMCID: VSports最新版本 - PMC3107102
- DOI: 10.1093/neuonc/nor042
Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma
Abstract (V体育ios版)
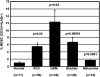
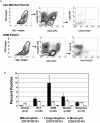
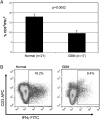
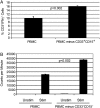
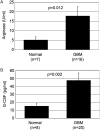
To assess the accumulation of myeloid-derived suppressor cells (MDSCs) in the peripheral blood of patients with glioma and to define their heterogeneity and their immunosuppressive function. Peripheral blood mononuclear cells (PBMCs) from healthy control subjects and from patients with newly diagnosed glioma were stimulated with anti-CD3/anti-CD28 and T cells assessed for intracellular expression of interferon (IFN)-γ. Antibody staining of PBMCs from glioma patients and healthy donors (CD33, HLADR, CD15, and CD14) followed by 4-color flow cytometry analysis-defined MDSC levels in the peripheral blood. To assess the role of MDSCs in suppressing T cell IFNγ production, PBMCs were depleted of MDSCs using anti-CD33 and anti-CD15 antibody-coated beads prior to T cell stimulation. Enzyme-linked immunosorbent assays were used to assess plasma arginase activity and the level of granulocyte colony-stimulating factor (G-CSF). Patients with glioblastoma have increased MDSC counts (CD33+HLADR-) in their blood that are composed of neutrophilic (CD15(+); >60%), lineage-negative (CD15(-)CD14(-); 31%), and monocytic (CD14(+); 6%) subsets. After stimulation, T cells from patients with glioblastoma had suppressed IFN-γ production when compared with healthy, age-matched donor T cells. Removal of MDSCs from the PBMCs with anti-CD33/CD15-coated beads significantly restored T cell function. Significant increases in arginase activity and G-CSF levels were observed in plasma specimens obtained from patients with glioblastoma. The accumulation of MDSCs in peripheral blood in patients with glioma likely promotes T cell immune suppression that is observed in this patient population. Increased plasma levels of arginase and G-CSF may relate to MDSC suppressor function and MDSC expansion, respectively, in patients with glioma VSports手机版. .
Figures
References
-
- Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59(10):1593–1600. doi:10.1007/s00262-010-0855-8. - DOI - PMC - PubMed
-
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. V体育安卓版 - doi:10.1038/nri2506. - DOI - PMC - PubMed
-
- Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother. 2001;24(6):431–446. VSports手机版 - doi:10.1097/00002371-200111000-00001. - DOI - PubMed
-
- Ugel S, Delpozzo F, Desantis G, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9(4):470–481. doi:10.1016/j.coph.2009.06.014. - DOI - PubMed
-
- Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. - V体育平台登录 - PubMed
Publication types
- VSports app下载 - Actions
MeSH terms
- Actions (VSports注册入口)
- "V体育官网" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- "VSports app下载" Actions
- V体育平台登录 - Actions
- V体育官网 - Actions
Substances
- V体育ios版 - Actions
Grants and funding (VSports最新版本)
LinkOut - more resources
VSports - Full Text Sources
Other Literature Sources (V体育官网入口)
"VSports最新版本" Research Materials

