mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry
- PMID: 21576258
- PMCID: PMC3110954
- DOI: "VSports手机版" 10.1101/gad.2042311
mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry (VSports最新版本)
Abstract
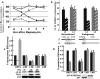
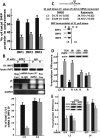
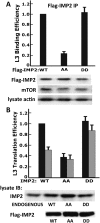
Variants in the IMP2 (insulin-like growth factor 2 [IGF2] mRNA-binding protein 2) gene are implicated in susceptibility to type 2 diabetes. We describe the ability of mammalian target of rapamycin (mTOR) to regulate the cap-independent translation of IGF2 mRNA through phosphorylation of IMP2, an oncofetal RNA-binding protein. IMP2 is doubly phosphorylated in a rapamycin-inhibitable, amino acid-dependent manner in cells and by mTOR in vitro. Double phosphorylation promotes IMP2 binding to the IGF2 leader 3 mRNA 5' untranslated region, and the translational initiation of this mRNA through eIF-4E- and 5' cap-independent internal ribosomal entry VSports手机版. Unexpectedly, the interaction of IMP2 with mTOR complex 1 occurs through mTOR itself rather than through raptor. Whereas depletion of mTOR strongly inhibits IMP2 phosphorylation in cells, comparable depletion of raptor has no effect; moreover, the ability of mTOR to phosphorylate IMP2 in vitro is unaffected by the elimination of raptor. Dual phosphorylation of IMP2 at the mTOR sites is evident in the mouse embryo, likely coupling nutrient sufficiency to IGF2 expression and fetal growth. Doubly phosphorylated IMP2 is also widely expressed in adult tissues, including islets of Langerhans. .
Figures




References
-
- Angel M, Yanik MF 2010. Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins. PLoS ONE 5: e11756 doi: 10.1371/journal.pone.0011756 - "V体育平台登录" PMC - PubMed
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 8: 73–82 - PubMed (VSports app下载)
-
- Barker DJ 2006. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49: 270–283 - "VSports在线直播" PubMed
-
- Brill LM, Salomon AR, Ficarro SB, Mukherji M, Stettler-Gill M, Peters EC 2004. Robust phosphoproteomic profiling of tyrosine phosphorylation sites from human T cells using immobilized metal affinity chromatography and tandem mass spectrometry. Anal Chem 76: 2763–2772 - PubMed (VSports手机版)
MeSH terms
- "VSports手机版" Actions
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- V体育官网 - Actions
- "VSports注册入口" Actions
- V体育官网 - Actions
- Actions (VSports app下载)
- "VSports app下载" Actions
- "VSports手机版" Actions
- V体育官网入口 - Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
- VSports在线直播 - Actions
Substances
- "VSports最新版本" Actions
- "V体育官网入口" Actions
- Actions (V体育ios版)
- Actions (VSports在线直播)
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
