CD8⁺ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung
- PMID: 21537083
- PMCID: VSports在线直播 - PMC3104744
- DOI: 10.1172/JCI44675 (VSports app下载)
CD8⁺ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung
Abstract
The human lung T cell compartment contains many CD8⁺ T cells specific for respiratory viruses, suggesting that the lung is protected from recurring respiratory infections by a resident T cell pool. The entry site for respiratory viruses is the epithelium, in which a subset of lung CD8⁺ T cells expressing CD103 (αE integrin) resides. Here, we determined the specificity and function of CD103⁺CD8⁺ T cells in protecting human lung against viral infection. Mononuclear cells were isolated from human blood and lung resection samples. Variable numbers of CD103⁺CD8⁺ T cells were retrieved from the lung tissue VSports手机版. Interestingly, expression of CD103 was seen only in lung CD8⁺ T cells specific for influenza but not in those specific for EBV or CMV. CD103⁺ and influenza-reactive cells preferentially expressed NKG2A, an inhibitor of CD8⁺ T cell cytotoxic function. In contrast to CD103⁻CD8⁺ T cells, most CD103⁺CD8⁺ cells did not contain perforin or granzyme B. However, they could quickly upregulate these cytotoxic mediators when exposed to a type I IFN milieu or via contact with their specific antigen. This mechanism may provide a rapid and efficient response to influenza infection, without inducing cytotoxic damage to the delicate epithelial barrier. .
Figures
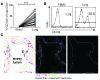


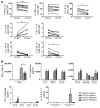

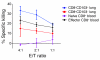
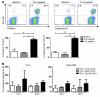
References
-
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159(11):5197–5200. - PubMed (V体育平台登录)
-
- Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol. 1999;163(10):5535–5543. - PubMed
Publication types
- VSports app下载 - Actions
MeSH terms
- "V体育平台登录" Actions
- "VSports" Actions
- VSports手机版 - Actions
- "V体育官网入口" Actions
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- V体育平台登录 - Actions
- "VSports最新版本" Actions
- V体育ios版 - Actions
Substances (V体育2025版)
- VSports手机版 - Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions

