VSports app下载 - Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases
- PMID: 21530742
- PMCID: PMC3773507
- DOI: "V体育平台登录" 10.1053/j.gastro.2011.02.016
Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases
Abstract
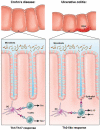
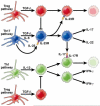
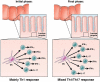
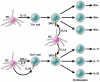
The cytokine responses characterizing the inflammatory bowel diseases are the key pathophysiologic elements that govern the initiation, evolution, and, ultimately, the resolution of these forms of inflammation. Studies during the last 2 decades now provide a detailed (but not yet complete) picture of the nature of these responses. The first tier of cytokine responses are governed by the T-cell differentiation patterns dominating the disease. In Crohn's disease, the major cytokines arise from T-helper cell (Th) 1 and Th17 CD4(+) T-cell differentiation and consist of interferon-γ and interleukin (IL)-17/IL-22 generated by these types of differentiation. The relative importance of these cytokines to Crohn's inflammation is still unclear, although evidence is mounting that interferon-γ is primus inter pare (first among equals). In contrast, in ulcerative colitis, a Th2-like differentiation process is paramount, which results in expansion of natural killer T cells producing IL-13 (and perhaps IL-5). These disease-specific cytokine patterns give rise to a second tier of cytokines that span the Th1/Th17-Th2 divide and act as upstream facilitators and downstream mediators of inflammation. These cytokines include the well-known tumor necrosis factor-α, IL-1β, IL-6 triumphirate, as well as a more recently studied cytokine known as TL1A (tumor necrosis factor-like ligand). In this review, we will explore this cytokine landscape with the view of providing an understanding of how recent and future anticytokine therapies actually function. VSports手机版.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved. V体育安卓版.
Figures

References
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. - PubMed
-
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. - PubMed
-
- Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1291–1290. - PMC (VSports注册入口) - PubMed
-
- Liu Z, Beboes K, Heremans H, Overbergh L, Mathieu C, Rutgeerts P, Ceuppens JL. Role of interleukin-12 in the induction of mucosal inflammation and abrogation of regulatory T cell function in chronic experimental colitis. Eur J Immunol. 2001;31:1550–1560. - PubMed
Publication types
MeSH terms
- V体育官网入口 - Actions
- VSports app下载 - Actions
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- V体育官网 - Actions
- Actions (V体育平台登录)
- V体育安卓版 - Actions
- "VSports app下载" Actions
Substances
- Actions (V体育平台登录)
"V体育官网入口" Grants and funding
LinkOut - more resources (V体育2025版)
VSports在线直播 - Full Text Sources
Other Literature Sources
"VSports最新版本" Medical
Research Materials

