Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy
- PMID: 21489257
- PMCID: PMC3098205
- DOI: 10.1186/1476-4598-10-37
Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy
Abstract
Background: Nrf2 is a key transcriptional regulator of a battery of genes that facilitate phase II/III drug metabolism and defence against oxidative stress. Nrf2 is largely regulated by Keap1, which directs Nrf2 for proteasomal degradation. The Nrf2/Keap1 system is dysregulated in lung, head and neck, and breast cancers and this affects cellular proliferation and response to therapy. Here, we have investigated the integrity of the Nrf2/Keap1 system in pancreatic cancer. VSports手机版.
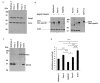
Results: Keap1, Nrf2 and the Nrf2 target genes AKR1c1 and GCLC were detected in a panel of five pancreatic cancer cell lines. Mutation analysis of NRF2 exon 2 and KEAP1 exons 2-6 in these cell lines identified no mutations in NRF2 and only synonomous mutations in KEAP1. RNAi depletion of Nrf2 caused a decrease in the proliferation of Suit-2, MiaPaca-2 and FAMPAC cells and enhanced sensitivity to gemcitabine (Suit-2), 5-flurouracil (FAMPAC), cisplatin (Suit-2 and FAMPAC) and gamma radiation (Suit-2) V体育安卓版. The expression of Nrf2 and Keap1 was also analysed in pancreatic ductal adenocarcinomas (n = 66 and 57, respectively) and matching normal benign epithelium (n = 21 cases). Whilst no significant correlation was seen between the expression levels of Keap1 and Nrf2 in the tumors, interestingly, Nrf2 staining was significantly greater in the cytoplasm of tumors compared to benign ducts (P < 0. 001). .
Conclusions: Expression of Nrf2 is up-regulated in pancreatic cancer cell lines and ductal adenocarcinomas. This may reflect a greater intrinsic capacity of these cells to respond to stress signals and resist chemotherapeutic interventions. Nrf2 also appears to support proliferation in certain pancreatic adenocarinomas. Therefore, strategies to pharmacologically manipulate the levels and/or activity of Nrf2 may have the potential to reduce pancreatic tumor growth, and increase sensitivity to therapeutics. V体育ios版.
Figures





References
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. et al.Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. j clin oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. - DOI - PubMed
-
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J. et al.Phase III Randomized Comparison of Gemcitabine Versus Gemcitabine Plus Capecitabine in Patients With Advanced Pancreatic Cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. - DOI (V体育平台登录) - PubMed
Publication types
MeSH terms
- "VSports手机版" Actions
- "VSports" Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
- Actions (VSports app下载)
Substances
- VSports注册入口 - Actions
- Actions (VSports app下载)
- V体育安卓版 - Actions
Grants and funding
"V体育ios版" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
"VSports" Miscellaneous

