"VSports注册入口" Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death
- PMID: 21475306
- PMCID: PMC3172118
- DOI: VSports最新版本 - 10.1038/cdd.2011.33
Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death
Abstract
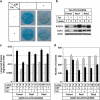
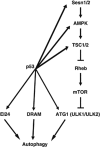
In yeast, activation of ATG1/ATG13 kinase complex initiates autophagy. This mechanism of autophagy initiation is conserved, as unc-51-like kinase 1 (ULK1) and unc-51-like kinase 2 (ULK2) are two mammalian functional homologues of ATG1 and form similar complex with mammalian ATG13. Here, we report that both ULK1 and ULK2 are transcriptional targets of tumor suppressor p53 VSports手机版. In response to DNA damage, ULK1 and ULK2 are upregulated by p53. The upregulation of ULK1 (ULK2)/ATG13 complex by p53 is necessary for the sustained autophagy activity induced by DNA damage. In this context, elevated autophagy contributes to subsequent cell death. These findings suggest that ULK1 and ULK2 may mediate part of tumor suppression activity in mammalian cells and contribute to the efficacy of genotoxic chemotherapeutic drugs. .
Figures




References (VSports)
-
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. - VSports - PubMed
-
- Meijer WH, van der Klei IJ, Veenhuis M, Kiel JA. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy. 2007;3:106–116. - PubMed
-
- Kuroyanagi H, Yan J, Seki N, Yamanouchi Y, Suzuki Y, Takano T, et al. Human ULK1, a novel serine/threonine kinase related to UNC-51 kinase of Caenorhabditis elegans: cDNA cloning, expression, and chromosomal assignment. Genomics. 1998;51:76–85. - PubMed
-
- Yan J, Kuroyanagi H, Kuroiwa A, Matsuda Y, Tokumitsu H, Tomoda T, et al. Identification of mouse ULK1, a novel protein kinase structurally related to C. elegans UNC-51. Biochem Biophys Res Commun. 1998;246:222–227. - PubMed
Publication types
- Actions (V体育官网)
MeSH terms
- Actions (VSports)
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- VSports - Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- Actions (VSports在线直播)
Substances
- "V体育2025版" Actions
- V体育官网入口 - Actions
- Actions (VSports在线直播)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous (V体育官网入口)

