The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth (V体育官网入口)
- PMID: 21445361
- PMCID: PMC3062567
- DOI: 10.1371/journal.pone.0018059
The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth
"VSports在线直播" Abstract
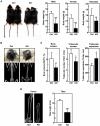
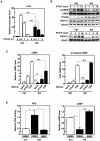
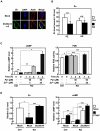
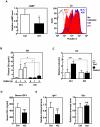
Aberrant zinc (Zn) homeostasis is associated with abnormal control of mammalian growth, although the molecular mechanisms of Zn's roles in regulating systemic growth remain to be clarified. Here we report that the cell membrane-localized Zn transporter SLC39A14 controls G-protein coupled receptor (GPCR)-mediated signaling. Mice lacking Slc39a14 (Slc39a14-KO mice) exhibit growth retardation and impaired gluconeogenesis, which are attributable to disrupted GPCR signaling in the growth plate, pituitary gland, and liver. The decreased signaling is a consequence of the reduced basal level of cyclic adenosine monophosphate (cAMP) caused by increased phosphodiesterase (PDE) activity in Slc39a14-KO cells. We conclude that SLC39A14 facilitates GPCR-mediated cAMP-CREB signaling by suppressing the basal PDE activity, and that this is one mechanism for Zn's involvement in systemic growth processes. Our data highlight SLC39A14 as an important novel player in GPCR-mediated signaling VSports手机版. In addition, the Slc39a14-KO mice may be useful for studying the GPCR-associated regulation of mammalian systemic growth. .
Conflict of interest statement
Figures



References
-
- Prasad AS. Zinc: an overview. Nutrition. 1995;11:93–99. - PubMed
-
- Vallee BL, Auld DS. New perspective on zinc biochemistry: cocatalytic sites in multi-zinc enzymes. Biochemistry. 1993;32:6493–6500. - PubMed
-
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. - V体育2025版 - PubMed
-
- Vallee BL. The function of metallothionein. Neurochem Int. 1995;27:23–33. - "VSports在线直播" PubMed
Publication types
"VSports app下载" MeSH terms
- Actions (VSports app下载)
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- VSports注册入口 - Actions
- V体育2025版 - Actions
Substances (V体育安卓版)
- Actions (VSports手机版)
- "V体育官网" Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
"V体育ios版" Research Materials

