Fbxw7-dependent degradation of Notch is required for control of "stemness" and neuronal-glial differentiation in neural stem cells
- PMID: 21349854
- PMCID: V体育官网入口 - PMC3075719
- DOI: 10.1074/jbc.M110.194936 (VSports app下载)
Fbxw7-dependent degradation of Notch is required for control of "stemness" and neuronal-glial differentiation in neural stem cells
Abstract
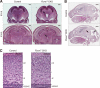
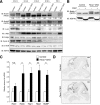
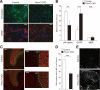
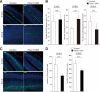
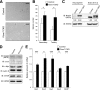
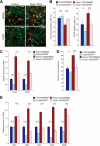
Control of the growth and differentiation of neural stem cells is fundamental to brain development and is largely dependent on the Notch signaling pathway VSports手机版. The mechanism by which the activity of Notch is regulated during brain development has remained unclear, however. Fbxw7 (also known as Fbw7, SEL-10, hCdc4, or hAgo) is the F-box protein subunit of an Skp1-Cul1-F-box protein (SCF)-type ubiquitin ligase complex that plays a central role in the degradation of Notch family members. We now show that mice with brain-specific deletion of Fbxw7 (Nestin-Cre/Fbxw7(F/F) mice) die shortly after birth with morphological abnormalities of the brain and the absence of suckling behavior. The maintenance of neural stem cells was sustained in association with the accumulation of Notch1 and Notch3, as well as up-regulation of Notch target genes in the mutant mice. Astrogenesis was also enhanced in the mutant mice in vivo, and the differentiation of neural progenitor cells was skewed toward astrocytes rather than neurons in vitro, with the latter effect being reversed by treatment of the cells with a pharmacological inhibitor of the Notch signaling pathway. Our results thus implicate Fbxw7 as a key regulator of the maintenance and differentiation of neural stem cells in the brain. .
Figures (VSports最新版本)

References
-
- Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999) Science 284, 770–776 - PubMed
-
- Justice N. J., Jan Y. N. (2002) Curr. Opin. Neurobiol. 12, 64–70 - PubMed
-
- Lai E. C. (2004) Development 131, 965–973 - PubMed
-
- Schuurmans C., Guillemot F. (2002) Curr. Opin. Neurobiol. 12, 26–34 - PubMed
-
- Furukawa T., Mukherjee S., Bao Z. Z., Morrow E. M., Cepko C. L. (2000) Neuron 26, 383–394 - PubMed (VSports)
Publication types
MeSH terms (VSports注册入口)
- "VSports在线直播" Actions
- "V体育ios版" Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
- Actions (VSports)
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- V体育安卓版 - Actions
- "V体育2025版" Actions
- VSports - Actions
- Actions (V体育平台登录)
"V体育2025版" Substances
- VSports注册入口 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- V体育官网入口 - Actions
LinkOut - more resources
Full Text Sources
VSports - Medical
Molecular Biology Databases
Miscellaneous

