Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis (V体育安卓版)
- PMID: 21319191
- PMCID: PMC3079544
- DOI: 10.1002/hep.24107
Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis
Abstract
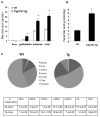
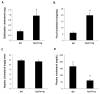
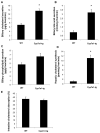
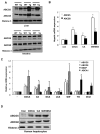
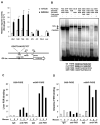
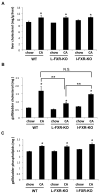
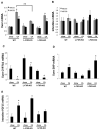
We reported previously that mice overexpressing cytochrome P450 7a1 (Cyp7a1; Cyp7a1-tg mice) are protected against high fat diet-induced hypercholesterolemia, obesity, and insulin resistance. Here, we investigated the underlying mechanism of bile acid signaling in maintaining cholesterol homeostasis in Cyp7a1-tg mice VSports手机版. Cyp7a1-tg mice had two-fold higher Cyp7a1 activity and bile acid pool than did wild-type mice. Gallbladder bile acid composition changed from predominantly cholic acid (57%) in wild-type to chenodeoxycholic acid (54%) in Cyp7a1-tg mice. Cyp7a1-tg mice had higher biliary and fecal cholesterol and bile acid secretion rates than did wild-type mice. Surprisingly, hepatic de novo cholesterol synthesis was markedly induced in Cyp7a1-tg mice but intestine fractional cholesterol absorption in Cyp7a1-tg mice remained the same as wild-type mice despite the presence of increased intestine bile acids. Interestingly, hepatic but not intestinal expression of several cholesterol (adenosine triphosphate-binding cassette G5/G8 [ABCG5/G8], scavenger receptor class B, member 1) and bile acid (ABCB11) transporters were significantly induced in Cyp7a1-tg mice. Treatment of mouse or human hepatocytes with a farnesoid X receptor (FXR) agonist GW4064 or bile acids induced hepatic Abcg5/g8 expression. A functional FXR binding site was identified in the Abcg5 gene promoter. Study of tissue-specific Fxr knockout mice demonstrated that loss of the Fxr gene in the liver attenuated bile acid induction of hepatic Abcg5/g8 and gallbladder cholesterol content, suggesting a role of FXR in the regulation of cholesterol transport. .
Conclusion: This study revealed a new mechanism by which increased Cyp7a1 activity expands the hydrophobic bile acid pool, stimulating hepatic cholesterol synthesis and biliary cholesterol secretion without increasing intestinal cholesterol absorption. This study demonstrated that Cyp7a1 plays a critical role in maintaining cholesterol homeostasis and underscores the importance of bile acid signaling in regulating overall cholesterol homeostasis. V体育安卓版.
Copyright © 2011 American Association for the Study of Liver Diseases. V体育ios版.
Conflict of interest statement
Figures
References
-
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. - PMC (V体育2025版) - PubMed
-
- Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, et al. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. - PMC (VSports注册入口) - PubMed
-
- Miyake JH, Duong-Polk XT, Taylor JM, Du EZ, Castellani LW, Lusis AJ, Davis RA. Transgenic Expression of Cholesterol 7α-Hydroxylase Prevents Atherosclerosis in C57BL/6J Mice. Arterioscler Thromb Vasc Biol. 2002;22:121–126. - PubMed
Publication types (V体育官网入口)
"VSports最新版本" MeSH terms
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- V体育2025版 - Actions
- "VSports" Actions
- V体育ios版 - Actions
Substances
- VSports注册入口 - Actions
- V体育安卓版 - Actions
- Actions (V体育安卓版)
- "V体育官网入口" Actions
- VSports手机版 - Actions
- Actions (V体育官网入口)
- "V体育ios版" Actions
- "V体育安卓版" Actions
V体育安卓版 - Grants and funding
LinkOut - more resources
VSports手机版 - Full Text Sources
Medical
"V体育官网" Miscellaneous
