FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation
- PMID: 21282377
- PMCID: PMC3039859 (V体育平台登录)
- DOI: 10.1084/jem.20100830
"V体育官网" FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation
Abstract
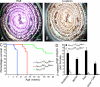
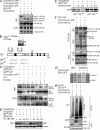
The Fbxw7 (F-box/WD repeat-containing protein 7; also called CDC4, Sel10, Ago, and Fbw7) component of the SCF (Skp1/Cullin/F-box protein) E3 ubiquitin ligase complex acts as a tumor suppressor in several tissues and targets multiple transcriptional activators and protooncogenes for ubiquitin-mediated degradation. To understand Fbxw7 function in the murine intestine, in this study, we specifically deleted Fbxw7 in the murine gut using Villin-Cre (Fbxw7(ΔG)). In wild-type mice, loss of Fbxw7 in the gut altered homeostasis of the intestinal epithelium, resulted in elevated Notch and c-Jun expression, and induced development of adenomas at 9-10 mo of age. In the context of APC (adenomatous polyposis coli) deficiency (Apc(Min/+) mice), loss of Fbxw7 accelerated intestinal tumorigenesis and death and promoted accumulation of β-catenin in adenomas at late but not early time points. At early time points, Fbxw7 mutant tumors showed accumulation of the DEK protooncogene. DEK expression promoted cell division and altered splicing of tropomyosin (TPM) RNA, which may also influence cell proliferation. DEK accumulation and altered TPM RNA splicing were also detected in FBXW7 mutant human colorectal tumor tissues. Given their reduced lifespan and increased incidence of intestinal tumors, Apc(Min/+)Fbxw7(ΔG) mice may be used for testing carcinogenicity and drug screening. VSports手机版.
Figures

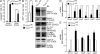




V体育官网 - References
-
- Alexiadis V., Waldmann T., Andersen J., Mann M., Knippers R., Gruss C. 2000. The protein encoded by the proto-oncogene DEK changes the topology of chromatin and reduces the efficiency of DNA replication in a chromatin-specific manner. Genes Dev. 14:1308–1312 - "V体育ios版" PMC - PubMed
-
- Bharadwaj S., Prasad G.L. 2002. Tropomyosin-1, a novel suppressor of cellular transformation is downregulated by promoter methylation in cancer cells. Cancer Lett. 183:205–213 10.1016/S0304-3835(02)00119-2 - VSports在线直播 - DOI - PubMed
-
- Bonetti P., Davoli T., Sironi C., Amati B., Pelicci P.G., Colombo E. 2008. Nucleophosmin and its AML-associated mutant regulate c-Myc turnover through Fbw7 γ. J. Cell Biol. 182:19–26 10.1083/jcb.200711040 - VSports - DOI - PMC - PubMed
Publication types
"VSports app下载" MeSH terms
- Actions (V体育官网)
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- Actions (VSports最新版本)
- V体育官网入口 - Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- "V体育官网入口" Actions
- V体育ios版 - Actions
- Actions (VSports app下载)
- V体育官网 - Actions
- VSports注册入口 - Actions
- VSports在线直播 - Actions
Substances
- V体育官网 - Actions
- V体育官网入口 - Actions
- VSports - Actions
- "VSports" Actions
- VSports在线直播 - Actions
- Actions (VSports)
Grants and funding (VSports)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases (V体育官网)
Miscellaneous

