"V体育2025版" An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion
- PMID: 21257711
- PMCID: PMC3057139
- DOI: 10.1158/0008-5472.CAN-10-1432
An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion
"VSports" Abstract
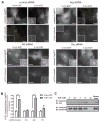
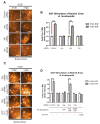
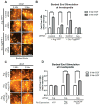
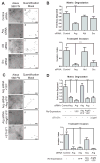
Invasive carcinoma cells use specialized actin polymerization-driven protrusions called invadopodia to degrade and possibly invade through the extracellular matrix (ECM) during metastasis VSports手机版. Phosphorylation of the invadopodium protein cortactin is a master switch that activates invadopodium maturation and function. Cortactin was originally identified as a hyperphosphorylated protein in v-Src-transformed cells, but the kinase or kinases that are directly responsible for cortactin phosphorylation in invadopodia remain unknown. In this study, we provide evidence that the Abl-related nonreceptor tyrosine kinase Arg mediates epidermal growth factor (EGF)-induced cortactin phosphorylation, triggering actin polymerization in invadopodia, ECM degradation, and matrix proteolysis-dependent tumor cell invasion. Both Src and Arg localize to invadopodia and are required for EGF-induced actin polymerization. Notably, Arg overexpression in Src knockdown cells can partially rescue actin polymerization in invadopodia while Src overexpression cannot compensate for loss of Arg, arguing that Src indirectly regulates invadopodium maturation through Arg activation. Our findings suggest a novel mechanism by which an EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Furthermore, they identify Arg as a novel mediator of invadopodia function and a candidate therapeutic target to inhibit tumor invasion in vivo. .
©2011 AACR.
Figures


References (VSports最新版本)
-
- Barsky SH, Siegal GP, Jannotta F, Liotta LA. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983;49:140–7. - PubMed
-
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–64. - PubMed
-
- Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. - PubMed
-
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–41. - PubMed
-
- Linder S. Invadosomes at a glance. J Cell Sci. 2009;122:3009–13. - PubMed
Publication types (V体育平台登录)
- Actions (V体育官网)
MeSH terms (V体育官网)
- "VSports app下载" Actions
- "V体育官网" Actions
- Actions (V体育官网)
- Actions (V体育官网)
- VSports最新版本 - Actions
- "V体育ios版" Actions
- V体育ios版 - Actions
- Actions (V体育官网)
Substances
- V体育ios版 - Actions
- Actions (V体育官网入口)
Grants and funding (V体育官网)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
VSports注册入口 - Research Materials
"V体育平台登录" Miscellaneous

