Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation
- PMID: 21228274
- PMCID: PMC3058966
- DOI: 10.1074/jbc.M110.202911
"V体育官网入口" Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation
Abstract
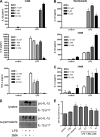
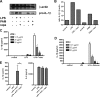
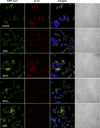
Autophagy is a key regulator of cellular homeostasis that can be activated by pathogen-associated molecules and recently has been shown to influence IL-1β secretion by macrophages. However, the mechanisms behind this are unclear. Here, we describe a novel role for autophagy in regulating the production of IL-1β in antigen-presenting cells. After treatment of macrophages with Toll-like receptor ligands, pro-IL-1β was specifically sequestered into autophagosomes, whereas further activation of autophagy with rapamycin induced the degradation of pro-IL-1β and blocked secretion of the mature cytokine. Inhibition of autophagy promoted the processing and secretion of IL-1β by antigen-presenting cells in an NLRP3- and TRIF-dependent manner. This effect was reduced by inhibition of reactive oxygen species but was independent of NOX2. Induction of autophagy in mice in vivo with rapamycin reduced serum levels of IL-1β in response to challenge with LPS. These data demonstrate that autophagy controls the production of IL-1β through at least two separate mechanisms: by targeting pro-IL-1β for lysosomal degradation and by regulating activation of the NLRP3 inflammasome. VSports手机版.
Figures





References
-
- Dinarello C. A. (2010) Eur. J. Immunol. 40, 599–606 - V体育官网 - PubMed
-
- Schroder K., Tschopp J. (2010) Cell 140, 821–832 - PubMed
-
- Eisenbarth S. C., Colegio O. R., O'Connor W., Sutterwala F. S., Flavell R. A. (2008) Nature 453, 1122–1126 - "VSports" PMC - PubMed
-
- Li H., Nookala S., Re F. (2007) J. Immunol. 178, 5271–5276 - PubMed
Publication types
- VSports手机版 - Actions
MeSH terms
- Actions (V体育官网入口)
- "V体育安卓版" Actions
- "V体育安卓版" Actions
- VSports - Actions
- "V体育官网入口" Actions
- V体育官网入口 - Actions
- VSports最新版本 - Actions
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- Actions (VSports)
- Actions (VSports最新版本)
- "V体育官网" Actions
- V体育安卓版 - Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
Substances
- Actions (VSports app下载)
- "V体育官网入口" Actions
- Actions (V体育ios版)
- Actions (V体育官网)
- "VSports手机版" Actions
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- Actions (V体育2025版)
- Actions (VSports手机版)
- "V体育安卓版" Actions
- Actions (VSports在线直播)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
VSports最新版本 - Molecular Biology Databases
Miscellaneous

