"V体育2025版" Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis
- PMID: 21220608
- PMCID: PMC4979099
- DOI: 10.1200/JCO.2010.32.8021
Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis
Abstract
Purpose: Myelofibrosis is a myeloid malignancy associated with anemia, splenomegaly, and constitutional symptoms. Patients frequently harbor JAK-STAT activating mutations that are sensitive to TG101348, a selective small-molecule Janus kinase 2 (JAK2) inhibitor VSports手机版. .
Patients and methods: In a multicenter phase I trial, oral TG101348 was administered once a day to patients with high- or intermediate-risk primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. V体育安卓版.
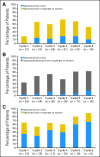
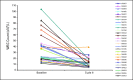
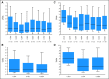
Results: Fifty-nine patients were treated, including 28 in the dose-escalation phase. The maximum-tolerated dose was 680 mg/d, and dose-limiting toxicity was a reversible and asymptomatic increase in the serum amylase level. Forty-three patients (73%) continued treatment beyond six cycles; the median cumulative exposure to TG101348 was 380 days V体育ios版. Adverse events included nausea, vomiting, diarrhea, anemia, and thrombocytopenia; corresponding grades 3 to 4 incidence rates were 3%, 3%, 10%, 35%, and 24%. TG101348 treatment had modest effect on serum cytokine levels, but greater than half of the patients with early satiety, night sweats, fatigue, pruritus, and cough achieved rapid and durable improvement in these symptoms. By six and 12 cycles of treatment, 39% and 47% of patients, respectively, had achieved a spleen response per International Working Group criteria. The majority of patients with leukocytosis or thrombocytosis at baseline (n = 28 and n = 10, respectively) achieved normalization of blood counts after six (57% and 90%, respectively) and 12 (56% and 88%, respectively) cycles. A significant decrease in JAK2 V617F allele burden was observed at 6 months in mutation-positive patients (n = 51; P = . 04), particularly in the subgroup with allele burden greater than 20% (n = 23; P < . 01); the decrease was durable at 12 months. .
Conclusion: TG101348 is well tolerated and produces significant reduction in disease burden and durable clinical benefit in patients with myelofibrosis VSports最新版本. .
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
"V体育官网入口" Figures

Comment in
-
Janus-activated kinase 2 inhibitors: a new era of targeted therapies providing significant clinical benefit for Philadelphia chromosome-negative myeloproliferative neoplasms.J Clin Oncol. 2011 Mar 1;29(7):781-3. doi: 10.1200/JCO.2010.33.4508. Epub 2011 Jan 10. J Clin Oncol. 2011. PMID: 21220594 No abstract available.
VSports注册入口 - References
-
- Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. - PubMed
-
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. - PubMed
-
- Hussein K, Pardanani AD, Van Dyke DL, et al. International Prognostic Scoring System– independent cytogenetic risk categorization in primary myelofibrosis. Blood. 2010;115:496–499. - "V体育安卓版" PubMed
-
- Patnaik MM, Caramazza D, Gangat N, et al. Age and platelet count are IPSS-independent prognostic factors in young patients with primary myelofibrosis and complement IPSS in predicting very long or very short survival. Eur J Haematol. 2010;84:105–108. - PubMed
-
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. - PubMed
Publication types
- VSports手机版 - Actions
MeSH terms
- VSports app下载 - Actions
- Actions (V体育ios版)
- V体育官网入口 - Actions
- Actions (VSports app下载)
- Actions (VSports)
- "VSports手机版" Actions
- V体育平台登录 - Actions
- "V体育安卓版" Actions
- VSports - Actions
- Actions (VSports app下载)
- "V体育ios版" Actions
- "V体育官网" Actions
- V体育官网 - Actions
- Actions (VSports)
- Actions (V体育2025版)
- "VSports在线直播" Actions
- V体育官网 - Actions
Substances
- V体育平台登录 - Actions
- Actions (V体育官网)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

