Phosphorylation meets nuclear import: a review
- PMID: 21182795
- PMCID: PMC3022542
- DOI: VSports最新版本 - 10.1186/1478-811X-8-32
Phosphorylation meets nuclear import: a review (VSports app下载)
Abstract
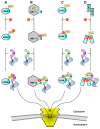
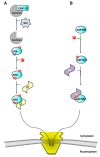
Phosphorylation is the most common and pleiotropic modification in biology, which plays a vital role in regulating and finely tuning a multitude of biological pathways. Transport across the nuclear envelope is also an essential cellular function and is intimately linked to many degeneration processes that lead to disease. It is therefore not surprising that phosphorylation of cargos trafficking between the cytoplasm and nucleus is emerging as an important step to regulate nuclear availability, which directly affects gene expression, cell growth and proliferation. However, the literature on phosphorylation of nucleocytoplasmic trafficking cargos is often confusing. Phosphorylation, and its mirror process dephosphorylation, has been shown to have opposite and often contradictory effects on the ability of cargos to be transported across the nuclear envelope. Without a clear connection between attachment of a phosphate moiety and biological response, it is difficult to fully understand and predict how phosphorylation regulates nucleocytoplasmic trafficking VSports手机版. In this review, we will recapitulate clue findings in the field and provide some general rules on how reversible phosphorylation can affect the nuclear-cytoplasmic localization of substrates. This is only now beginning to emerge as a key regulatory step in biology. .
Figures


References
-
- Mosammaparast N, Pemberton LF. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004;14:547–556. doi: 10.1016/j.tcb.2004.09.004. - "V体育平台登录" DOI - PubMed
-
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. - DOI (V体育官网) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources (VSports注册入口)
Other Literature Sources

