Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death (V体育ios版)
- PMID: 21173155
- PMCID: PMC3048716
- DOI: "V体育ios版" 10.1074/jbc.M110.197301
Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death
Abstract
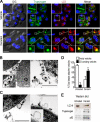
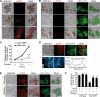
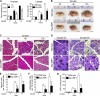
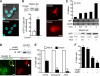
Autophagy has recently elicited significant attention as a mechanism that either protects or promotes cell death, although different autophagy pathways, and the cellular context in which they occur, remain to be elucidated. We report a thorough cellular and biochemical characterization of a novel selective autophagy that works as a protective cell response. This new selective autophagy is activated in pancreatic acinar cells during pancreatitis-induced vesicular transport alteration to sequester and degrade potentially deleterious activated zymogen granules. We have coined the term "zymophagy" to refer to this process VSports手机版. The autophagy-related protein VMP1, the ubiquitin-protease USP9x, and the ubiquitin-binding protein p62 mediate zymophagy. Moreover, VMP1 interacts with USP9x, indicating that there is a close cooperation between the autophagy pathway and the ubiquitin recognition machinery required for selective autophagosome formation. Zymophagy is activated by experimental pancreatitis in genetically engineered mice and cultured pancreatic acinar cells and by acute pancreatitis in humans. Furthermore, zymophagy has pathophysiological relevance by controlling pancreatitis-induced intracellular zymogen activation and helping to prevent cell death. Together, these data reveal a novel selective form of autophagy mediated by the VMP1-USP9x-p62 pathway, as a cellular protective response. .
V体育官网入口 - Figures




References
-
- Kraft C., Peter M., Hofmann K. (2010) Nat. Cell Biol. 12, 836–841 - PubMed
-
- Suzuki K., Ohsumi Y. (2007) FEBS Lett. 581, 2156–2161 - PubMed
-
- Kraft C., Reggiori F., Peter M. (2009) Biochim. Biophys. Acta 1793, 1404–1412 - PubMed (V体育ios版)
-
- Pankiv S., Clausen T. H., Lamark T., Brech A., Bruun J. A., Outzen H., Øvervatn A., Bjørkøy G., Johansen T. (2007) J. Biol. Chem. 282, 24131–24145 - PubMed
-
- Kim P. K., Hailey D. W., Mullen R. T., Lippincott-Schwartz J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20567–20574 - "V体育安卓版" PMC - PubMed
Publication types
"V体育平台登录" MeSH terms
- Actions (V体育安卓版)
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- Actions (VSports手机版)
- "V体育2025版" Actions
- V体育2025版 - Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- V体育ios版 - Actions
- Actions (V体育ios版)
- V体育ios版 - Actions
- V体育安卓版 - Actions
V体育2025版 - Substances
- Actions (V体育平台登录)
- "V体育平台登录" Actions
VSports注册入口 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

