TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells
- PMID: 21084298
- PMCID: PMC3023530
- DOI: 10.1074/jbc.M110.175109
TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells
Abstract (VSports)
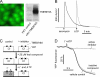
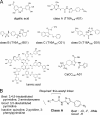
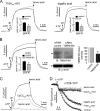
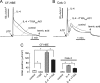
TMEM16A (ANO1) functions as a calcium-activated chloride channel (CaCC). We developed pharmacological tools to investigate the contribution of TMEM16A to CaCC conductance in human airway and intestinal epithelial cells. A screen of ∼110,000 compounds revealed four novel chemical classes of small molecule TMEM16A inhibitors that fully blocked TMEM16A chloride current with an IC(50) < 10 μM, without interfering with calcium signaling. Following structure-activity analysis, the most potent inhibitor, an aminophenylthiazole (T16A(inh)-A01), had an IC(50) of ∼1 μM. Two distinct types of inhibitors were identified. Some compounds, such as tannic acid and the arylaminothiophene CaCC(inh)-A01, fully inhibited CaCC current in human bronchial and intestinal cells VSports手机版. Other compounds, including T16A(inh)-A01 and digallic acid, inhibited total CaCC current in these cells poorly, but blocked mainly an initial, agonist-stimulated transient chloride current. TMEM16A RNAi knockdown also inhibited mainly the transient chloride current. In contrast to the airway and intestinal cells, all TMEM16A inhibitors fully blocked CaCC current in salivary gland cells. We conclude that TMEM16A carries nearly all CaCC current in salivary gland epithelium, but is a minor contributor to total CaCC current in airway and intestinal epithelia. The small molecule inhibitors identified here permit pharmacological dissection of TMEM16A/CaCC function and are potential development candidates for drug therapy of hypertension, pain, diarrhea, and excessive mucus production. .
Figures



References
-
- Hartzell C., Putzier I., Arreola J. (2005) Annu. Rev. Physiol. 67, 719–758 - "VSports app下载" PubMed
-
- Eggermont J. (2004) Proc. Am. Thorac. Soc. 1, 22–27 - PubMed
-
- Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) Nature 455, 1210–1215 - "V体育ios版" PubMed
-
- Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) Science 322, 590–594 - "V体育平台登录" PubMed
"VSports注册入口" Publication types
- "V体育官网入口" Actions
- Actions (VSports最新版本)
MeSH terms
- Actions (VSports注册入口)
- VSports app下载 - Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- "VSports" Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- "VSports手机版" Actions
- "VSports" Actions
- Actions (VSports注册入口)
V体育安卓版 - Substances
- "VSports最新版本" Actions
- Actions (VSports手机版)
- "VSports" Actions
Grants and funding
- "VSports手机版" HL73856/HL/NHLBI NIH HHS/United States
- "V体育ios版" R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- V体育2025版 - DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States (VSports)
- "V体育ios版" R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- "V体育官网入口" RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- "V体育2025版" R37 DK035124/DK/NIDDK NIH HHS/United States
- "VSports app下载" R37 EB000415/EB/NIBIB NIH HHS/United States
VSports - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

