Synthesis, characterization, and biological evaluation of poly(L-γ-glutamyl-glutamine)- paclitaxel nanoconjugate
- PMID: 21042550
- PMCID: PMC2964040
- DOI: 10.2147/IJN.S13482
"V体育官网" Synthesis, characterization, and biological evaluation of poly(L-γ-glutamyl-glutamine)- paclitaxel nanoconjugate
Abstract
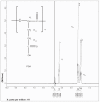
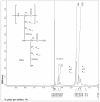
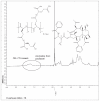
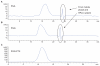
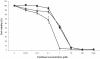
The purpose of this study was to develop a novel, highly water-soluble poly(L-γ-glutamyl-glutamine)-paclitaxel nanoconjugate (PGG-PTX) that would improve the therapeutic index of paclitaxel (PTX). PGG-PTX is a modification of poly(L-glutamic acid)- paclitaxel conjugate (PGA-PTX) in which an additional glutamic acid has been added to each glutamic side chain in the polymer. PGG-PTX has higher water-solubility and faster dissolution than PGA-PTX. Unlike PGA-PTX, PGG-PTX self-assembles into nanoparticles, whose size remains in the range of 12-15 nm over the concentration range from 25 to 2,000 μg/mL in saline. Its critical micellar concentration in saline was found to be ~25 μg/mL. The potency of PGG-PTX when tested in vitro against the human lung cancer H460 cell line was comparable to other known polymer-PTX conjugates. However, PGG-PTX possesses lower toxicity compared with PGA-PTX in mice. The maximum tolerated dose of PGG-PTX was found to be 350 mg PTX/kg, which is 2 VSports手机版. 2-fold higher than the maximum tolerated dose of 160 mg PTX/kg reported for the PGA-PTX. This result indicates that PGG-PTX was substantially less toxic in vivo than PGA-PTX. .
Keywords: anticancer; nanoconjugates; nanoparticles; poly(L-glutamic acid); poly(L-γ-glutamyl-glutamine)-paclitaxel. V体育安卓版.
Figures






References
-
- diMasi JA, Hansen RW, Grabowki HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. - PubMed
-
- Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–287. - PubMed
-
- Ansari MJ, Kohli K, Dixit N. Microemulsions as potential drug delivery systems: a review. PDA J Pharm Sci Technol. 2008;62:66–79. - PubMed
-
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. - PubMed
-
- Haag R, Kratz F. Polymer therapeutics: concept and applications. Angew Chem Int Ed Engl. 2006;45:1198–1215. - PubMed
VSports手机版 - Publication types
- "V体育官网入口" Actions
MeSH terms
- Actions (V体育2025版)
- Actions (VSports app下载)
- VSports手机版 - Actions
- V体育官网入口 - Actions
- V体育2025版 - Actions
- "VSports手机版" Actions
- Actions (VSports最新版本)
- VSports手机版 - Actions
- "V体育2025版" Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
- Actions (VSports手机版)
- "V体育安卓版" Actions
Substances (VSports在线直播)
- "VSports" Actions
LinkOut - more resources
Full Text Sources
"VSports app下载" Other Literature Sources

