Thermochemistry of proton-coupled electron transfer reagents and its implications
- PMID: 20925411
- PMCID: PMC3006073
- DOI: 10.1021/cr100085k
Thermochemistry of proton-coupled electron transfer reagents and its implications (V体育安卓版)
V体育平台登录 - Erratum in
-
Correction to Thermochemistry of Proton-Coupled Electron Transfer Reagents and its Implications.Chem Rev. 2022 Jan 12;122(1):1482. doi: 10.1021/acs.chemrev.1c00791. Epub 2021 Oct 12. Chem Rev. 2022. PMID: 34637292 Free PMC article. No abstract available.
"VSports" Figures

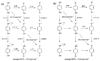
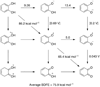
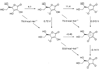


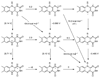

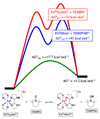
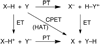

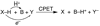
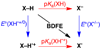

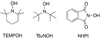

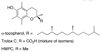
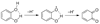
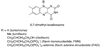



References (VSports手机版)
-
- Huynh MHV, Meyer TJ. Chem. Rev. 2007;107:5004. - PMC (V体育安卓版) - PubMed
- Meyer TJ, Huynh MHV. Inorg. Chem. 2003;42:8140. - PubMed
-
- Hynes JT, Klinman JP, Limback H-H, Schowen RL, editors. Hydrogen-Transfer Reactions. Weinheim: Wiley-VCH; 2007.
-
- Costentin C. Chem. Rev. 2008;108:2145. - VSports app下载 - PubMed
-
- Stock JS, Orna MV, editors. Electrochemistry, past and present. Washington, DC: American Chemical Society; 1989. ACS Symposium Series 390.
-
-
Cf., Appel AM, Lee S-J, Franz JA, DuBois DL, DuBois MR. J. Am. Chem. Soc. 2009;131:5224.
-
Publication types
- "VSports注册入口" Actions
MeSH terms
- "VSports app下载" Actions
- Actions (V体育安卓版)
- VSports手机版 - Actions
Substances
- VSports注册入口 - Actions
"V体育官网入口" Grants and funding
LinkOut - more resources
Full Text Sources

