Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species
- PMID: 20861446
- PMCID: PMC2955141
- DOI: 10.1073/pnas.1012016107
V体育2025版 - Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species
"V体育安卓版" Abstract
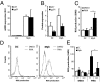
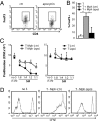
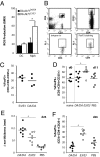
The phagocyte NAPDH-oxidase complex consists of several phagocyte oxidase (phox) proteins, generating reactive oxygen species (ROS) upon activation VSports手机版. ROS are involved in the defense against microorganisms and also in immune regulation. Defective ROS formation leads to chronic granulomatous disease (CGD) with increased incidence of autoimmunity and disturbed resolution of inflammation. Because regulatory T cells (Tregs) suppress autoimmune T-cell responses and are crucial in down-regulating immune responses, we hypothesized that ROS deficiency may lead to decreased Treg induction. Previously, we showed that in p47(phox)-mutated mice, reconstitution of macrophages (Mph) with ROS-producing capacity was sufficient to protect the mice from arthritis. Now, we present evidence that Mph-derived ROS induce Tregs. In vitro, we showed that Mph ROS-dependently induce Treg, using an NADPH-oxidase inhibitor. This finding was confirmed genetically: rat or human CGD Mph with mutated p47(phox) or gp91(phox) displayed hampered Treg induction and T-cell suppression. However, basal Treg numbers in these subjects were comparable to those in controls, indicating a role for ROS in induction of peripheral Tregs. Induction of allogeneic delayed-type hypersensitivity with p47(phox)-mutated Mph confirmed the importance of Mph-derived ROS in Treg induction in vivo. We conclude that NAPDH oxidase activity in Mph is important for the induction of Tregs to regulate T cell-mediated inflammation. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures
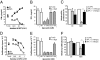

References
-
- Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009;30:201–208. - PubMed
-
- Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: From the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. - "V体育2025版" PubMed
-
- Gelderman KA, et al. Rheumatoid arthritis: The role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal. 2007;9:1541–1567. - PubMed
Publication types (VSports手机版)
VSports最新版本 - MeSH terms
- VSports app下载 - Actions
- Actions (VSports)
- VSports手机版 - Actions
- "VSports" Actions
- Actions (V体育安卓版)
- V体育ios版 - Actions
- VSports手机版 - Actions
- Actions (VSports手机版)
- "VSports app下载" Actions
Substances
- Actions (VSports)
- "VSports" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- VSports app下载 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

