Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis
- PMID: 20833380
- PMCID: PMC2952357
- DOI: 10.1016/j.chom.2010.08.004
Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis
Abstract
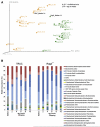
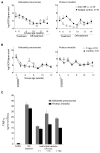
Disruption of homeostasis between the host immune system and the intestinal microbiota leads to inflammatory bowel disease (IBD) VSports手机版. Whether IBD is instigated by individual species or disruptions of entire microbial communities remains controversial. We characterized the fecal microbial communities in the recently described T-bet(-/-) ×Rag2(-/-) ulcerative colitis (TRUC) model driven by T-bet deficiency in the innate immune system. 16S rRNA-based analysis of TRUC and Rag2(-/-) mice revealed distinctive communities that correlate with host genotype. The presence of Klebsiella pneumoniae and Proteus mirabilis correlates with colitis in TRUC animals, and these TRUC-derived strains can elicit colitis in Rag2(-/-) and WT adults but require a maternally transmitted endogenous microbial community for maximal intestinal inflammation. Cross-fostering experiments indicated a role for these organisms in maternal transmission of disease. Our findings illustrate how gut microbial communities work in concert with specific culturable colitogenic agents to cause IBD. .
Copyright © 2010 Elsevier Inc V体育安卓版. All rights reserved. .
Figures


References
-
- Buescher ES, Malinowska I. Soluble receptors and cytokine antagonists in human milk. Pediatr Res. 1996;40:839–844. - PubMed
-
- Cerrutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. - VSports注册入口 - PMC - PubMed
-
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. - "VSports注册入口" PMC - PubMed
Publication types
V体育官网入口 - MeSH terms
- V体育官网 - Actions
- VSports - Actions
- "V体育安卓版" Actions
- V体育ios版 - Actions
- Actions (VSports app下载)
- Actions (VSports手机版)
- "VSports在线直播" Actions
- "V体育官网" Actions
- V体育安卓版 - Actions
- Actions (VSports)
- VSports在线直播 - Actions
- "V体育ios版" Actions
- V体育官网 - Actions
Substances
- V体育官网入口 - Actions
Grants and funding
LinkOut - more resources (VSports在线直播)
VSports app下载 - Full Text Sources
Other Literature Sources
Medical

