Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-γ in a nitric oxide-dependent manner (V体育2025版)
- PMID: 20805415
- PMCID: PMC4346245
- DOI: "V体育平台登录" 10.4049/jimmunol.1001778
Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-γ in a nitric oxide-dependent manner
Abstract
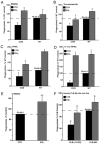
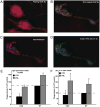
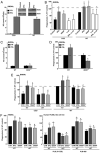
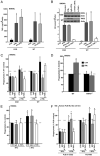
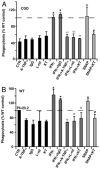
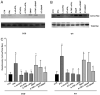
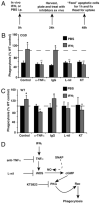
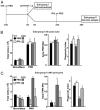
Immunodeficiency in chronic granulomatous disease (CGD) is well characterized. Less understood are exaggerated sterile inflammation and autoimmunity associated with CGD. Impaired recognition and clearance of apoptotic cells resulting in their disintegration may contribute to CGD inflammation. We hypothesized that priming of macrophages (Ms) with IFN-γ would enhance impaired engulfment of apoptotic cells in CGD. Diverse M populations from CGD (gp91(phox)(-/-)) and wild-type mice, as well as human Ms differentiated from monocytes and promyelocytic leukemia PLB-985 cells (with and without mutation of the gp91(phox)), demonstrated enhanced engulfment of apoptotic cells in response to IFN-γ priming. Priming with IFN-γ was also associated with increased uptake of Ig-opsonized targets, latex beads, and fluid phase markers, and it was accompanied by activation of the Rho GTPase Rac. Enhanced Rac activation and phagocytosis following IFN-γ priming were dependent on NO production via inducible NO synthase and activation of protein kinase G. Notably, endogenous production of TNF-α in response to IFN-γ priming was critically required for inducible NO synthase upregulation, NO production, Rac activation, and enhanced phagocytosis. Treatment of CGD mice with IFN-γ also enhanced uptake of apoptotic cells by M in vivo via the signaling pathway. Importantly, during acute sterile peritonitis, IFN-γ treatment reduced excess accumulation of apoptotic neutrophils and enhanced phagocytosis by CGD Ms. These data support the hypothesis that in addition to correcting immunodeficiency in CGD, IFN-γ priming of Ms restores clearance of apoptotic cells and may thereby contribute to resolution of exaggerated CGD inflammation VSports手机版. .
"V体育平台登录" Figures
References
-
- Gallin JI, Buescher ES. Abnormal regulation of inflammatory skin responses in male patients with chronic granulomatous disease. Inflammation. 1983;7:227–232. - PubMed
-
- Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. - PubMed
-
- Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. - PubMed
-
- Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. - PubMed
-
- Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. - PubMed (V体育2025版)
"V体育2025版" Publication types
MeSH terms
- Actions (VSports最新版本)
- VSports在线直播 - Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
- "VSports手机版" Actions
Substances
- Actions (VSports)
Grants and funding
LinkOut - more resources
VSports - Full Text Sources
Molecular Biology Databases
Miscellaneous

