VSports - Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1
- PMID: 20727791
- PMCID: VSports最新版本 - PMC2928475
- DOI: V体育安卓版 - 10.1016/j.immuni.2010.08.002
Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1
"V体育平台登录" Abstract
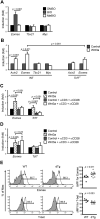
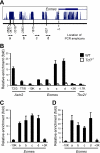
T cell factor 1 (TCF-1) is a transcription factor known to act downstream of the canonical Wnt pathway and is essential for normal T cell development. However, its physiological roles in mature CD8(+) T cell responses are unknown. Here we showed that TCF-1 deficiency limited proliferation of CD8(+) effector T cells and impaired their differentiation toward a central memory phenotype. Moreover, TCF-1-deficient memory CD8(+) T cells were progressively lost over time, exhibiting reduced expression of the antiapoptotic molecule Bcl-2 and interleukin-2 receptor beta chain and diminished IL-15-driven proliferation. TCF-1 was directly associated with the Eomes allele and the Wnt-TCF-1 pathway was necessary and sufficient for optimal Eomes expression in naive and memory CD8(+) T cells VSports手机版. Importantly, forced expression of Eomes partly protected TCF-1-deficient memory CD8(+) T cells from time-dependent attrition. Our studies thus identify TCF-1 as a critical player in a transcriptional program that regulates memory CD8 differentiation and longevity. .
Copyright 2010 Elsevier Inc V体育安卓版. All rights reserved. .
Figures





Comment in
-
TCF-1 flips the switch on Eomes.Immunity. 2010 Aug 27;33(2):145-7. doi: 10.1016/j.immuni.2010.08.008. Immunity. 2010. PMID: 20732636
References
-
- Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. - V体育ios版 - PubMed
-
- Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. - PubMed
-
- Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. - PubMed
-
- Bianchi T, Gasser S, Trumpp A, MacDonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. Blood. 2006;107:3992–3999. - PubMed
Publication types
- VSports在线直播 - Actions
MeSH terms
- "V体育平台登录" Actions
- Actions (V体育官网)
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- "V体育官网" Actions
- "V体育ios版" Actions
- "VSports在线直播" Actions
- "VSports app下载" Actions
- Actions (VSports手机版)
- VSports手机版 - Actions
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
Substances
- V体育2025版 - Actions
- Actions (V体育ios版)
- VSports在线直播 - Actions
- Actions (VSports在线直播)
- Actions (V体育官网入口)
Associated data
- Actions
Grants and funding
- R01 AI050073/AI/NIAID NIH HHS/United States
- R01 AI083286/AI/NIAID NIH HHS/United States
- R01 AI059752/AI/NIAID NIH HHS/United States
- AI077504/AI/NIAID NIH HHS/United States (V体育官网入口)
- AI080966/AI/NIAID NIH HHS/United States
- R21 AI042767/AI/NIAID NIH HHS/United States
- R21 AI077504/AI/NIAID NIH HHS/United States
- "V体育ios版" R01 AI046653/AI/NIAID NIH HHS/United States
- "VSports在线直播" R37 AI042767/AI/NIAID NIH HHS/United States
- "V体育2025版" R21 AI080966/AI/NIAID NIH HHS/United States
- HL095540/HL/NHLBI NIH HHS/United States
- VSports注册入口 - AI083286/AI/NIAID NIH HHS/United States
- "VSports在线直播" R01 AI042767/AI/NIAID NIH HHS/United States
- "V体育官网入口" AI050073/AI/NIAID NIH HHS/United States
- VSports - AI046653/AI/NIAID NIH HHS/United States
- AI042767/AI/NIAID NIH HHS/United States (VSports手机版)
- AI059752/AI/NIAID NIH HHS/United States
- R01 HL095540/HL/NHLBI NIH HHS/United States
LinkOut - more resources (V体育2025版)
Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育2025版)
Research Materials

