Intestinal goblet cells and mucins in health and disease: recent insights and progress
- PMID: 20703838
- PMCID: PMC2933006 (VSports)
- DOI: 10.1007/s11894-010-0131-2
Intestinal goblet cells and mucins in health and disease: recent insights and progress
Abstract
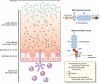
The mucus layer coating the gastrointestinal tract is the front line of innate host defense, largely because of the secretory products of intestinal goblet cells. Goblet cells synthesize secretory mucin glycoproteins (MUC2) and bioactive molecules such as epithelial membrane-bound mucins (MUC1, MUC3, MUC17), trefoil factor peptides (TFF), resistin-like molecule beta (RELMbeta), and Fc-gamma binding protein (Fcgbp). The MUC2 mucin protein forms trimers by disulfide bonding in cysteine-rich amino terminal von Willebrand factor (vWF) domains, coupled with crosslinking provided by TFF and Fcgbp proteins with MUC2 vWF domains, resulting in a highly viscous extracellular layer. Colonization by commensal intestinal microbiota is limited to an outer "loose" mucus layer, and interacts with the diverse oligosaccharides of mucin glycoproteins, whereas an "inner" adherent mucus layer is largely devoid of bacteria. Defective mucus layers resulting from lack of MUC2 mucin, mutated Muc2 mucin vWF domains, or from deletion of core mucin glycosyltransferase enzymes in mice result in increased bacterial adhesion to the surface epithelium, increased intestinal permeability, and enhanced susceptibility to colitis caused by dextran sodium sulfate. Changes in mucin gene expression and mucin glycan structures occur in cancers of the intestine, contributing to diverse biologic properties involved in the development and progression of cancer. Further research is needed on identification and functional significance of various components of mucus layers and the complex interactions among mucus layers, microbiota, epithelial cells, and the underlying innate and adaptive immunity. Further elucidation of the regulatory mechanisms involved in mucin changes in cancer and inflammation may lead to the development of novel therapeutic approaches VSports手机版. .
VSports手机版 - Figures
V体育ios版 - References
-
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. - DOI (V体育平台登录) - PubMed
-
- Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. - PubMed
Publication types
- "V体育平台登录" Actions
- V体育ios版 - Actions
- "V体育平台登录" Actions
V体育ios版 - MeSH terms
- VSports注册入口 - Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- VSports app下载 - Actions
- Actions (VSports最新版本)
- "VSports" Actions
- "VSports在线直播" Actions
- V体育ios版 - Actions
- V体育官网入口 - Actions
- Actions (VSports app下载)
- "V体育安卓版" Actions
- "V体育ios版" Actions
- "VSports在线直播" Actions
- "V体育2025版" Actions
Substances
- "VSports app下载" Actions
- "VSports在线直播" Actions
"V体育ios版" Grants and funding
"V体育官网入口" LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育平台登录" Research Materials
Miscellaneous

