Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation
- PMID: 20675303
- PMCID: PMC3050595
- DOI: 10.1677/JOE-10-0181
Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation
Abstract
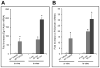
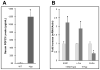
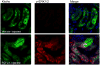
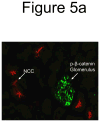
Fibroblast growth factor-23 (FGF23), a hormone central to renal phosphate handling, is elevated in multiple hypophosphatemic disorders. Initial FGF23-dependent Erk1/2 activity in the kidney localizes to the distal convoluted tubule (DCT) with the co-receptor α-Klotho (KL), distinct from Npt2a in proximal tubules (PT). The Hyp mouse model of X-linked hypophosphatemic rickets (XLH) is characterized by hypophosphatemia with increased Fgf23, and patients with XLH elevate FGF23 following combination therapy of phosphate and calcitriol. The molecular signaling underlying renal FGF23 activity, and whether these pathways are altered in hypophosphatemic disorders, is unknown. To examine Npt2a in vivo, mice were injected with FGF23. Initial p-Erk1/2 activity in the DCT occurred within 10 min; however, Npt2a protein was latently reduced in the PT at 30-60 min, and was independent of Npt2a mRNA changes. KL-null mice had no DCT p-Erk1/2 staining following FGF23 delivery. Under basal conditions in Hyp mice, c-Fos and Egr1, markers of renal Fgf23 activity, were increased; however, KL mRNA was reduced 60% (P<0 VSports手机版. 05). Despite the prevailing hypophosphatemia and elevated Fgf23, FGF23 injections into Hyp mice activated p-Erk1/2 in the DCT. FGF23 injection also resulted in phospho-β-catenin (p-β-cat) co-localization with KL in wild-type mice, and Hyp mice demonstrated strong p-β-cat staining under basal conditions, indicating potential crosstalk between mitogen-activated protein kinase and Wnt signaling. Collectively, these studies refine the mechanisms for FGF23 bioactivity, and demonstrate novel suppression of Wnt signaling in a KL-dependent DCT-PT axis, which is likely altered in XLH. Finally, the current treatment of phosphate and calcitriol for hypophosphatemic disorders may increase FGF23 activity. .
Conflict of interest statement
EF, LS, SD, JM, and DE, have nothing to declare V体育安卓版. SS is employed by Genzyme, Corp. KW receives research funding from Genzyme, and royalties for licensing the FGF23 gene to Kyowa Hakko Kirin, Ltd.
Figures






References
-
- Azam N, Zhang MY, Wang X, Tenenhouse HS, Portale AA. Disordered regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase gene expression by phosphorus in X-linked hypophosphatemic (hyp) mice. Endocrinology. 2003;144:3463–3468. - "VSports最新版本" PubMed
-
- Bachar-Dahan L, Goltzmann J, Yaniv A, Gazit A. Engrailed-1 negatively regulates beta-catenin transcriptional activity by destabilizing beta-catenin via a glycogen synthase kinase-3beta-independent pathway. Mol Biol Cell. 2006;17:2572–2580. - V体育ios版 - PMC - PubMed
-
- Bacic D, Schulz N, Biber J, Kaissling B, Murer H, Wagner CA. Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Arch. 2003;446:52–60. - PubMed (V体育安卓版)
-
- Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol. 1998;9:1347–1358. - PubMed
-
- Custer M, Lotscher M, Biber J, Murer H, Kaissling B. Expression of Na-P(i) cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol. 1994;266:F767–774. - PubMed
Publication types
- V体育安卓版 - Actions
- Actions (V体育官网)
MeSH terms
- Actions (V体育官网)
- "V体育ios版" Actions
- "V体育安卓版" Actions
- Actions (VSports)
- VSports最新版本 - Actions
- "VSports" Actions
- Actions (V体育2025版)
- "V体育官网入口" Actions
- Actions (VSports注册入口)
- V体育平台登录 - Actions
- Actions (V体育官网入口)
- Actions (V体育官网)
- Actions (VSports在线直播)
- V体育安卓版 - Actions
Substances
- "VSports app下载" Actions
- Actions (VSports注册入口)
- Actions (VSports手机版)
- Actions (V体育平台登录)
- Actions (V体育安卓版)
Grants and funding
"VSports在线直播" LinkOut - more resources
Full Text Sources
V体育ios版 - Miscellaneous

