Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer
- PMID: 20664591
- PMCID: PMC2938261
- DOI: "V体育官网入口" 10.1038/sj.bjc.6605819
Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer
Abstract
Background: The purpose of this work was to determine the efficacy of inhibiting mammalian target of rapamycin (mTOR) in pancreatic cancer preclinical models and translate preclinical observations to the clinic. VSports手机版.
Methods: Temsirolimus (20 mg Kg(-1) daily) was administered to freshly generated pancreatic cancer xenografts. Tumour growth inhibition was determined after 28 days. Xenografts were characterised at baseline by gene expression and comparative genomic hybridisation. Patients with advanced, gemcitabine-resistant pancreatic cancer were treated with sirolimus (5 mg daily). The primary end point was 6-month survival rate (6mSR). Correlative studies included immunohistochemistry assessment of pathway expression in baseline tumours, drug pharmacokinetics (PKs), response assessment by FDG-PET and pharmacodynamic effects in peripheral-blood mononuclear cells (PBMCs). V体育安卓版.
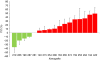
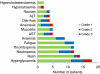
Results: In all, 4 of 17 xenografts (23%) responded to treatment. Sensitive tumours were characterised by gene copy number variations and overexpression of genes leading to activation of the PI3K/Akt/mTOR pathway. Activation of p70S6K correlated with drug activity in the preclinical studies V体育ios版. Sirolimus was well tolerated in the clinic, showed predictable PKs, exerted pathway inhibition in post-treatment PBMCs and resulted in a 6mSR of 26%. No correlation, however, was found between activated p70S6K in tumour tissues and anti-tumour effects. .
Conclusion: Sirolimus activity in pancreatic cancer was marginal and not predicted by the selected biomarker VSports最新版本. .
Figures (V体育2025版)


VSports手机版 - References
-
- Agbunag C, Bar-Sagi D (2004) Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res 64: 5659–5663 - PubMed
-
- Berger DP, Fiebig HH, Winterhalter BR, Wallbrecher E, Henss H (1990) Preclinical phase II study of ifosfamide in human tumour xenografts in vivo. Cancer Chemother Pharmacol 26(Suppl): S7–S11 - PubMed
-
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL (2008) Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med 5: e8. - PMC - PubMed
-
- Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, Dancey J, Hidalgo M, Walsh DJ (2005) Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 23: 5294–5304 - PubMed
V体育官网入口 - Publication types
MeSH terms
- V体育官网 - Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions
- V体育官网 - Actions
- V体育官网 - Actions
- V体育官网入口 - Actions
- "VSports手机版" Actions
Substances
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- "VSports app下载" Actions
LinkOut - more resources
Full Text Sources
"VSports在线直播" Other Literature Sources
Medical
Miscellaneous

