Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children's Oncology Group study
- PMID: 20660830
- PMCID: PMC2940398
- DOI: 10.1200/JCO.2009.27.5016
Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children's Oncology Group study (V体育平台登录)
Abstract
Purpose: Single-agent topotecan (TOPO) and combination topotecan and cyclophosphamide (TOPO/CTX) were compared in a phase II randomized trial in relapsed/refractory neuroblastoma VSports手机版. Because responders often underwent further therapies, novel statistical methods were required to compare the long-term outcome of the two treatments. .
Patients and methods: Children with refractory/recurrent neuroblastoma (only one prior aggressive chemotherapy regimen) were randomly assigned to daily 5-day topotecan (2 mg/m(2)) or combination topotecan (0. 75 mg/m(2)) and cyclophosphamide (250 mg/m(2)). A randomized two-stage group sequential design enrolled 119 eligible patients. Toxicity and response were estimated V体育安卓版. Long-term outcome of protocol therapy was assessed using novel methods-causal inference-which allowed adjustment for the confounding effect of off-study therapies. .
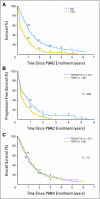
Results: Seven more responses were observed for TOPO/CTX (complete response [CR] plus partial response [PR], 18 [32%] of 57) than TOPO (CR+PR, 11 [19%] of 59;P = . 081); toxicity was similar. At 3 years, progression-free survival (PFS) and overall survival (OS) were 4% +/- 2% and 15% +/- 4%, respectively. PFS was significantly better for TOPO/CTX (P = . 029); there was no difference in OS. Older age at diagnosis and lack of MYCN amplification predicted increased OS (P < . 05) V体育ios版. Adjusting for randomized treatment effect and subsequent autologous stem-cell transplantation, there was no difference between TOPO and TOPO/CTX in terms of the proportion alive at 2 years. .
Conclusion: TOPO/CTX was superior to TOPO in terms of PFS, but there was no OS difference. After adjustment for subsequent therapies, no difference was detected in the proportion alive at 2 years. Causal inference methods for assessing long-term outcomes of phase II therapies after subsequent treatment can elucidate effects of initial therapies. VSports最新版本.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
"VSports在线直播" Figures
Comment in
-
Causal inference for definitive clinical end points in a randomized clinical trial with intervening nonrandomized treatments.J Clin Oncol. 2010 Aug 20;28(24):3800-2. doi: 10.1200/JCO.2010.30.0442. Epub 2010 Jul 26. J Clin Oncol. 2010. PMID: 20660828 No abstract available.
References
-
- Takimoto CH, Arbuck SG. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. Topoisomerase I targeting agents: The camptothecins, in Chabner BA, Longo DL (eds): Cancer Chemotherapy and Biotherapy; pp. 579–604.
-
- Oguro M. In Proceedings of The Third Conference on DNA Topoisomerases in Therapy. New York: 1990. Oct 15-18, A topoisomerase I inhibitor, CPT-11: Its enigmatic antitumor activity in combination with other agents in vitro; p. 35.
-
- Blaney SM, Balis FM, Cole DE, et al. Pediatric phase I trial and pharmacokinetic study of topotecan administered as a 24-hour continuous infusion. Cancer Res. 1993;53:1032–1036. - PubMed
-
- Tubergen DG, Stewart CF, Pratt CB, et al. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1996;18:352–361. - PubMed
-
- Cole D, Blaney S, Balis F, et al. A phase I and pharmacokinetic study of topotecan in pediatric patients. Proc Am Soc Clin Oncol. 1992;11:116. abstr XXXX.
Publication types
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- "VSports最新版本" Actions
VSports最新版本 - MeSH terms
- Actions (VSports注册入口)
- "V体育官网入口" Actions
- "V体育官网入口" Actions
- VSports app下载 - Actions
- Actions (VSports)
- V体育ios版 - Actions
- "V体育安卓版" Actions
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- Actions (VSports在线直播)
- "VSports app下载" Actions
Substances
- Actions (VSports注册入口)
- Actions (VSports)
Grants and funding (VSports app下载)
LinkOut - more resources
Full Text Sources
Medical
"V体育平台登录" Research Materials

