Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres
- PMID: 20651253
- PMCID: PMC2922592
- DOI: 10.1073/pnas.1008850107
Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres
Abstract
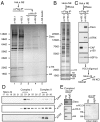
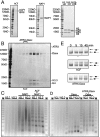
The histone variant H3. 3 is implicated in the formation and maintenance of specialized chromatin structure in metazoan cells. H3. 3-containing nucleosomes are assembled in a replication-independent manner by means of dedicated chaperone proteins. We previously identified the death domain associated protein (Daxx) and the alpha-thalassemia X-linked mental retardation protein (ATRX) as H3. 3-associated proteins. Here, we report that the highly conserved N terminus of Daxx interacts directly with variant-specific residues in the H3. 3 core. Recombinant Daxx assembles H3. 3/H4 tetramers on DNA templates, and the ATRX-Daxx complex catalyzes the deposition and remodeling of H3. 3-containing nucleosomes. We find that the ATRX-Daxx complex is bound to telomeric chromatin, and that both components of this complex are required for H3. 3 deposition at telomeres in murine embryonic stem cells (ESCs). These data demonstrate that Daxx functions as an H3 VSports手机版. 3-specific chaperone and facilitates the deposition of H3. 3 at heterochromatin loci in the context of the ATRX-Daxx complex. .
Conflict of interest statement
The authors declare no conflict of interest.
"V体育平台登录" Figures


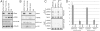
"VSports最新版本" References
-
- Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328(5982):1161–1164. - "V体育官网入口" PMC - PubMed
-
- Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol. 2008;10(1):102–109. - PubMed
-
- Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002;9(6):1191–1200. - "VSports最新版本" PubMed
VSports注册入口 - Publication types
- "VSports最新版本" Actions
- Actions (VSports在线直播)
MeSH terms
- Actions (VSports在线直播)
- Actions (VSports app下载)
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- "V体育官网" Actions
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- Actions (V体育2025版)
- VSports - Actions
- "V体育官网" Actions
- "V体育平台登录" Actions
Substances (V体育官网)
- VSports在线直播 - Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- "V体育官网" Actions
- VSports app下载 - Actions
Grants and funding (VSports手机版)
LinkOut - more resources
Full Text Sources
"V体育官网入口" Other Literature Sources
Molecular Biology Databases

