"VSports手机版" ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis
- PMID: 20637199
- PMCID: PMC2967588
- DOI: 10.1053/j.gastro.2010.07.006
ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis
Abstract
Background & aims: The identification of numerous genes that confer susceptibility to Crohn's disease (CD) indicates that this complex disease might arise from alterations in several genes with related functions VSports手机版. We examined the functional interaction between the CD risk genes ATG16L1 and NOD2 to identify an autophagy-dependent pathway that is altered by disease-associated variants. .
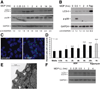
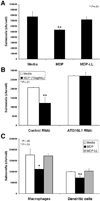
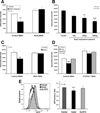
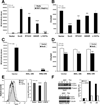
Methods: We assessed Nod2 signaling and autophagy activation in response to muramyl dipeptide (MDP) by immunoblot, confocal microscopy, flow cytometry, reporter gene, and gentamicin protection assays in human epithelial cell lines and primary human macrophages and dendritic cells from healthy individuals V体育安卓版. The requirement of Nod2 and ATG16L1 expression and the effects of CD-associated variants in MDP-stimulated autophagy and Nod2-dependent signaling were assessed in cell lines manipulated by RNA interference, inhibitors, or ATG16L1 or NOD2 variants and in primary macrophages and dendritic cells from healthy genotyped donors. .
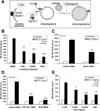
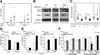
Results: MDP stimulation of epithelial cells, macrophages, and dendritic cells activated autophagy and nuclear factor κB and mitogen-activated protein kinase signaling; it also increased killing of Salmonella V体育ios版. These responses depended on ATG16L1 and Nod2 expression and were impaired by CD-associated NOD2 variants. Nod2-dependent signaling was not impaired in cells with the ATG16L1 T300A genotype, which is associated with CD. However, the ATG16L1 T300A variant blocked the increase in MDP-mediated killing of Salmonella only in epithelial cell lines and not primary macrophages or dendritic cells. .
Conclusions: ATG16L1 and NOD2 are components of an autophagy-mediated antibacterial pathway that is altered in a cell- and function-specific manner by CD-associated mutations. VSports最新版本.
Copyright © 2010 AGA Institute. Published by Elsevier Inc V体育平台登录. All rights reserved. .
Conflict of interest statement
Figures
Comment in
-
Crohn's disease susceptibility gene interactions, a NOD to the newcomer ATG16L1.Gastroenterology. 2010 Nov;139(5):1448-50. doi: 10.1053/j.gastro.2010.09.023. Epub 2010 Sep 25. Gastroenterology. 2010. PMID: 20875485 No abstract available.
References
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. - VSports最新版本 - PubMed
-
- Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771–774. - PubMed
-
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. - "V体育安卓版" PubMed
-
- Inohara N, Chamaillard M, McDonald C, et al. NOD-LRR proteins: role in microbial-host interactions and inflammatory disease. Ann Rev Biochem. 2005;74:355–383. - V体育官网入口 - PubMed
-
- Abraham C, Cho JH. Functional consequences of NOD2 (CARD15) mutations. Inflamm Bowel Dis. 2006;12:641–650. - PubMed
Publication types (VSports手机版)
- "V体育2025版" Actions
- Actions (VSports在线直播)
MeSH terms (VSports最新版本)
- Actions (VSports在线直播)
- "V体育官网入口" Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
- Actions (V体育官网入口)
- V体育平台登录 - Actions
- Actions (V体育平台登录)
- "V体育ios版" Actions
- Actions (V体育官网)
- "VSports注册入口" Actions
Substances
- VSports - Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

