The ubiquitin ligase Fbxw7 controls adipocyte differentiation by targeting C/EBPalpha for degradation
- PMID: 20534483
- PMCID: PMC2900639
- DOI: 10.1073/pnas.0913367107 (V体育ios版)
The ubiquitin ligase Fbxw7 controls adipocyte differentiation by targeting C/EBPalpha for degradation
Abstract
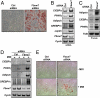
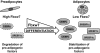
Adipose tissue controls body lipid and energy metabolism, as well as food intake, and abnormalities in adipose function play a central role in diseases such as obesity and type-2 diabetes. Adipocyte differentiation is controlled by a transcriptional cascade involving PPARgamma and members of the C/EBP family of transcription factors. Here, we demonstrate that C/EBPalpha is targeted for degradation by the ubiquitin ligase Fbxw7 in a phosphorylation-dependent manner. Importantly, inactivation of Fbxw7 is sufficient to convert mouse preadipocytes into mature adipocytes in a manner dependent on C/EBPalpha. In addition, inactivation of Fbxw7 promotes adipocyte differentiation of human adult stem cells. Taken together, our results suggest that Fbxw7 is a negative regulator of adipogenesis by targeting C/EBPalpha for degradation. This notion is supported by the observation that the expression of Fbxw7 is down-regulated during adipocyte differentiation, resulting in the accumulation of proadipogenic proteins such as C/EBPalpha. Thus, Fbxw7 could be an important regulator of energy and lipid metabolism VSports手机版. .
Conflict of interest statement (V体育平台登录)
The authors declare no conflict of interest.
Figures
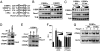


References
-
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. - "V体育官网入口" PMC - PubMed
-
- Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. - "V体育官网" PubMed
-
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. - PubMed
-
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. - PubMed
VSports手机版 - Publication types
- Actions (V体育ios版)
V体育ios版 - MeSH terms
- "V体育ios版" Actions
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- "VSports在线直播" Actions
- V体育官网 - Actions
- VSports app下载 - Actions
- Actions (VSports手机版)
- VSports在线直播 - Actions
- "VSports最新版本" Actions
- Actions (VSports最新版本)
- Actions (V体育2025版)
Substances
- VSports注册入口 - Actions
- VSports手机版 - Actions
- "VSports手机版" Actions
- "V体育平台登录" Actions
- V体育平台登录 - Actions
"V体育安卓版" LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

