Characterization of disease-related 5beta-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency (V体育官网)
- PMID: 20522910
- PMCID: PMC2915689
- DOI: 10.1074/jbc.M110.127779
Characterization of disease-related 5beta-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency
Abstract
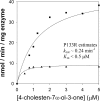
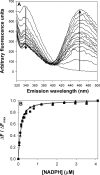
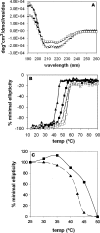
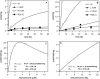
Bile acid deficiency is a serious syndrome in newborns that can result in death if untreated. 5beta-Reductase deficiency is one form of bile acid deficiency and is characterized by dramatically decreased levels of physiologically active 5beta-reduced bile acids VSports手机版. AKR1D1 (aldo-keto reductase 1D1) is the only known human enzyme that stereo-specifically reduces the Delta(4) double bond in 3-keto steroids and sterols to yield the 5beta-hydrogenated product. Analysis of the AKR1D1 gene in five patients with 5beta-reductase deficiency revealed five different mutations resulting in an amino acid substitution in the protein. To investigate a causal role for these observed point mutations in AKR1D1 in 5beta-reductase deficiency, we characterized their effect on enzymatic properties. Attempts to purify mutant enzymes by overexpression in Escherichia coli only yielded sufficient amounts of the P133R mutant for further characterization. This enzyme displayed a highly reduced K(m) and V(max) reminiscent of uncompetitive kinetics with 4-cholesten-7alpha-ol-3-one as substrate. In addition, this mutant displayed no change in cofactor affinity but was more thermolabile in the absence of NADPH as judged by CD spectroscopy. All mutants were compared following expression in HEK 293 cells. Although these enzymes were equally expressed based on mRNA levels, protein expression and functional activity were dramatically reduced. Cycloheximide treatment also revealed that several of the expressed mutants were less stable. Our findings show that the reported mutations in AKR1D1 in patients with 5beta-reductase lead to significantly decreased levels of active enzyme and could be causal in the development of bile acid deficiency syndrome. .
Figures


References
-
- Kondo K. H., Kai M. H., Setoguchi Y., Eggertsen G., Sjöblom P., Setoguchi T., Okuda K. I., Björkhem I. (1994) Eur. J. Biochem. 219, 357–363 - PubMed
-
- Deslypere J. P., Young M., Wilson J. D., McPhaul M. J. (1992) Mol. Cell. Endocrinol. 88, 15–22 - PubMed
-
- Moore L. B., Parks D. J., Jones S. A., Bledsoe R. K., Consler T. G., Stimmel J. B., Goodwin B., Liddle C., Blanchard S. G., Willson T. M., Collins J. L., Kliewer S. A. (2000) J. Biol. Chem. 275, 15122–15127 - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- V体育官网 - Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions
- V体育官网 - Actions
- "VSports最新版本" Actions
- V体育官网 - Actions
Substances
- "V体育官网入口" Actions
- V体育官网 - Actions
- "V体育平台登录" Actions
- VSports - Actions
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
VSports app下载 - Full Text Sources
Molecular Biology Databases
"V体育官网" Miscellaneous

