VSports最新版本 - Early signals during CD8 T cell priming regulate the generation of central memory cells
- PMID: 20519649
- PMCID: "VSports注册入口" PMC2997352
- DOI: 10.4049/jimmunol.1000492 (V体育平台登录)
V体育平台登录 - Early signals during CD8 T cell priming regulate the generation of central memory cells
VSports手机版 - Abstract
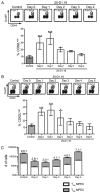
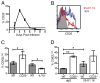
The CD8(+) T cell response to infection is characterized by the appearance of short-lived (CD127(low) killer cell lectin-like receptor G 1-high) and memory-precursor (CD127(high) killer cell lectin-like receptor G 1-low) effector cells. How and when central-memory T (T(CM); CD62L(high) CCR7(+)) cell and effector-memory T(T(EM); CD62L(low) CCR7(-)) cell subsets are established remains unclear. We now show that the T(CM) cell lineage represents an early developmental branchpoint during the CD8(+) T cell response to infection. Central-memory CD8(+) T cells could be identified prior to the peak of the CD8(+) T cell response and were enriched in lymphoid organs VSports手机版. Moreover, the kinetics and magnitude of T(CM) cell development were dependent on the infectious agent. Furthermore, the extent of early Ag availability, which regulated programmed death-1 and CD25 expression levels, controlled the T(CM)/T(EM) cell lineage decision ultimately through IL-2 and IL-15 signaling levels. These observations identify key early signals that help establish the T(CM)/T(EM) cell dichotomy and provide the means to manipulate memory lineage choices. .
Conflict of interest statement
Figures
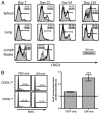
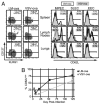
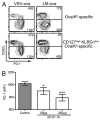
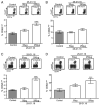
References
-
- Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, von Herrath MG, Buchmeier MJ, Grey H, Sette A. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–2133. - PMC (VSports注册入口) - PubMed
-
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. - PubMed
Publication types
- Actions (V体育官网)
MeSH terms
- VSports - Actions
- "VSports app下载" Actions
- VSports手机版 - Actions
- VSports在线直播 - Actions
- "V体育平台登录" Actions
- VSports最新版本 - Actions
- VSports app下载 - Actions
- Actions (VSports注册入口)
- "V体育ios版" Actions
- "VSports注册入口" Actions
- "VSports" Actions
- VSports最新版本 - Actions
"V体育2025版" Substances
Grants and funding
"V体育官网" LinkOut - more resources
Full Text Sources
"VSports最新版本" Other Literature Sources
Molecular Biology Databases (VSports在线直播)
Research Materials

