Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease
- PMID: 20512988
- PMCID: PMC2920131
- DOI: 10.1002/hep.23599
Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease
Abstract
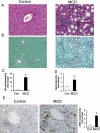
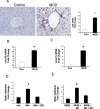
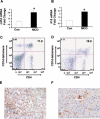
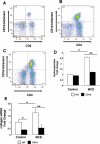
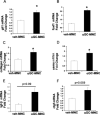
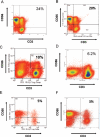
Liver inflammation is greater in nonalcoholic steatohepatitis (NASH) than steatosis, suggesting that immune responses contribute to nonalcoholic fatty liver disease (NAFLD) progression. Livers normally contain many natural killer T (NKT) cells that produce factors that modulate inflammatory and fibrogenic responses. Such cells are relatively depleted in steatosis, but their status in more advanced NAFLD is uncertain VSports手机版. We hypothesized that NKT cells accumulate and promote fibrosis progression in NASH. We aimed to determine if livers become enriched with NKT cells during NASH-related fibrosis; identify responsible mechanisms; and assess if NKT cells stimulate fibrogenesis. NKT cells were analyzed in wildtype mice and Patched-deficient (Ptc(+/-)) mice with an overly active Hedgehog (Hh) pathway, before and after feeding methionine choline-deficient (MCD) diets to induce NASH-related fibrosis. Effects of NKT cell-derived factors on hepatic stellate cells (HSC) were examined and fibrogenesis was evaluated in CD1d-deficient mice that lack NKT cells. NKT cells were quantified in human cirrhotic and nondiseased livers. During NASH-related fibrogenesis in wildtype mice, Hh pathway activation occurred, leading to induction of factors that promoted NKT cell recruitment, retention, and viability, plus liver enrichment with NKT cells. Ptc(+/-) mice accumulated more NKT cells and developed worse liver fibrosis; CD1d-deficient mice that lack NKT cells were protected from fibrosis. NKT cell-conditioned medium stimulated HSC to become myofibroblastic. Liver explants were 2-fold enriched with NKT cells in patients with non-NASH cirrhosis, and 4-fold enriched in patients with NASH cirrhosis. .
Conclusion: Hh pathway activation leads to hepatic enrichment with NKT cells that contribute to fibrosis progression in NASH. V体育安卓版.
Figures

V体育安卓版 - References
-
- Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–378. - PubMed (V体育平台登录)
-
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. - PubMed
-
- Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. - PubMed
-
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. - PubMed
-
- Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. Hepatology. 2004;40:1033–1040. - PubMed
Publication types
MeSH terms
- Actions (VSports最新版本)
- VSports - Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- VSports在线直播 - Actions
- Actions (VSports在线直播)
Substances
- "VSports" Actions
Grants and funding
"VSports app下载" LinkOut - more resources
Full Text Sources
Other Literature Sources
