"VSports最新版本" Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation
- PMID: 20389017
- PMCID: PMC2860902
- DOI: 10.1172/JCI41072
Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation (V体育官网)
VSports最新版本 - Abstract
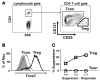
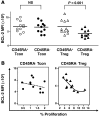
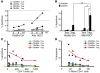
CD4+CD25+Foxp3+ Tregs have an indispensable role in the maintenance of tolerance after allogeneic HSC transplantation (HSCT) VSports手机版. Patients with chronic graft-versus-host disease (GVHD) have fewer circulating Tregs, but the mechanisms that lead to this deficiency of Tregs after HSCT are not known. Here, we analyzed reconstitution of Tregs and conventional CD4+ T cells (Tcons) in patients who underwent allogeneic HSCT after myeloablative conditioning. Following transplant, thymic generation of naive Tregs was markedly impaired, and reconstituting Tregs had a predominantly activated/memory phenotype. In response to CD4+ lymphopenia after HSCT, Tregs underwent higher levels of proliferation than Tcons, but Tregs undergoing homeostatic proliferation also showed increased susceptibility to Fas-mediated apoptosis. Prospective monitoring of CD4+ T cell subsets revealed that Tregs rapidly expanded and achieved normal levels by 9 months after HSCT, but Treg levels subsequently declined in patients with prolonged CD4+ lymphopenia. This resulted in a relative deficiency of Tregs, which was associated with a high incidence of extensive chronic GVHD. These studies indicate that CD4+ lymphopenia is a critical factor in Treg homeostasis and that prolonged imbalance of Treg homeostasis after HSCT can result in loss of tolerance and significant clinical disease manifestations. .
Figures



V体育安卓版 - References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. - PubMed (VSports最新版本)
Publication types
MeSH terms
- Actions (V体育ios版)
- Actions (V体育平台登录)
- "V体育官网" Actions
- V体育ios版 - Actions
- Actions (VSports)
- "VSports app下载" Actions
- "VSports手机版" Actions
- "V体育ios版" Actions
"VSports app下载" Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

